Crosstalk between Wnt and bone morphogenetic protein signaling during osteogenic differentiation
- PMID: 38455105
- PMCID: PMC10915952
- DOI: 10.4252/wjsc.v16.i2.102
Crosstalk between Wnt and bone morphogenetic protein signaling during osteogenic differentiation
Abstract
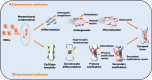
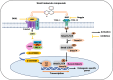
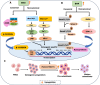
Mesenchymal stem cells (MSCs) originate from many sources, including the bone marrow and adipose tissue, and differentiate into various cell types, such as osteoblasts and adipocytes. Recent studies on MSCs have revealed that many transcription factors and signaling pathways control osteogenic development. Osteogenesis is the process by which new bones are formed; it also aids in bone remodeling. Wnt/β-catenin and bone morphogenetic protein (BMP) signaling pathways are involved in many cellular processes and considered to be essential for life. Wnt/β-catenin and BMPs are important for bone formation in mammalian development and various regulatory activities in the body. Recent studies have indicated that these two signaling pathways contribute to osteogenic differentiation. Active Wnt signaling pathway promotes osteogenesis by activating the downstream targets of the BMP signaling pathway. Here, we briefly review the molecular processes underlying the crosstalk between these two pathways and explain their participation in osteogenic differentiation, emphasizing the canonical pathways. This review also discusses the crosstalk mechanisms of Wnt/BMP signaling with Notch- and extracellular-regulated kinases in osteogenic differentiation and bone development.
Keywords: Bone; Bone morphogenetic proteins; Mesenchymal stem cells; Osteogenic differentiation; Wnt/β-catenin.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures
Similar articles
-
Ginkgo biloba extract promotes osteogenic differentiation of human bone marrow mesenchymal stem cells in a pathway involving Wnt/β-catenin signaling.Pharmacol Res. 2015 Jul;97:70-8. doi: 10.1016/j.phrs.2015.04.004. Epub 2015 Apr 23. Pharmacol Res. 2015. PMID: 25917209
-
PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells.Curr Stem Cell Res Ther. 2016;11(3):216-25. doi: 10.2174/1574888x10666150519093429. Curr Stem Cell Res Ther. 2016. PMID: 25986621 Review.
-
Effect of TAK1 on osteogenic differentiation of mesenchymal stem cells by regulating BMP-2 via Wnt/β-catenin and MAPK pathway.Organogenesis. 2018 Jan 2;14(1):36-45. doi: 10.1080/15476278.2018.1455010. Organogenesis. 2018. PMID: 29913119 Free PMC article.
-
Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway.Toxicol Lett. 2016 Jan 5;240(1):68-80. doi: 10.1016/j.toxlet.2015.10.007. Epub 2015 Oct 22. Toxicol Lett. 2016. PMID: 26478571
-
Signaling network regulating osteogenesis in mesenchymal stem cells.J Cell Commun Signal. 2022 Mar;16(1):47-61. doi: 10.1007/s12079-021-00635-1. Epub 2021 Jul 8. J Cell Commun Signal. 2022. PMID: 34236594 Free PMC article. Review.
Cited by
-
β-Catenin: A Key Molecule in Osteoblast Differentiation.Biomolecules. 2025 Jul 18;15(7):1043. doi: 10.3390/biom15071043. Biomolecules. 2025. PMID: 40723914 Free PMC article. Review.
-
Anti-Microbial Drug Metronidazole Promotes Fracture Healing: Enhancement in the Bone Regenerative Efficacy of the Drug by a Biodegradable Sustained-Release In Situ Gel Formulation.Biomedicines. 2024 Jul 18;12(7):1603. doi: 10.3390/biomedicines12071603. Biomedicines. 2024. PMID: 39062176 Free PMC article.
-
MIR155HG suppresses the osteogenic differentiation of bone marrow mesenchymal stem cells through regulating miR-155-5p and DKK1 expression.J Orthop Surg Res. 2025 Apr 19;20(1):392. doi: 10.1186/s13018-025-05798-w. J Orthop Surg Res. 2025. PMID: 40251598 Free PMC article.
-
Synergistic Crosstalk of PACAP and Notch Signaling Pathways in Bone Development.Int J Mol Sci. 2025 May 26;26(11):5088. doi: 10.3390/ijms26115088. Int J Mol Sci. 2025. PMID: 40507899 Free PMC article.
-
Challenges and future perspectives in using mesenchymal stem cells for efficient bone fracture healing.Front Bioeng Biotechnol. 2025 May 30;13:1568914. doi: 10.3389/fbioe.2025.1568914. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40521093 Free PMC article. Review.
References
-
- Black JD, Tadros BJ. Bone structure: from cortical to calcium. Orthop Trauma. 2020;34:113–119.
-
- van Dijk Christiansen P, Andreasen CM, El-Masri BM, Laursen KS, Delaisse JM, Andersen TL. Osteoprogenitor recruitment and differentiation during intracortical bone remodeling of adolescent humans. Bone. 2023;177:116896. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

