Extent of κ-casein hydrolysis during renneting of bovine milk: A critical assessment of the analytical and estimation approaches
- PMID: 38455171
- PMCID: PMC10916671
- DOI: 10.1002/fsn3.3868
Extent of κ-casein hydrolysis during renneting of bovine milk: A critical assessment of the analytical and estimation approaches
Abstract
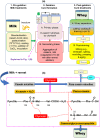
Renneting is an enzymatic process that turns milk into curd which is then transformed into cheese. Rennet-induced coagulation of caseins (CNs) is the critical step during this process and the key is the primary hydrolysis of κ-CN's Phe105-Met106 bond by chymosin. This article comprehensively reviews the existing data on the extent/degree of κ-CN hydrolysis during renneting of bovine milk and critically evaluates its determination methods. The data show that under normal cheese-making conditions, milk gelation occurs at a degree of κ-CN hydrolysis <80%, which varies due to several factors including analytical and estimation approaches. The common approach involves isolating the macropeptides released, by precipitating whey proteins and residual CN in 1%-12% trichloroacetic acid (TCA), then assuming that the maximum amount obtained is 100% κ-CN hydrolysis. The drawback is that the estimated degree of κ-CN hydrolysis may be higher than the actual value as TCA partially precipitates the macropeptide fractions. Moreover, macropeptide isolation seems unnecessary based on current advances in chromatographic and electrophoretic techniques. The present work proposes a simple mass balance-based approach that will provide accurate estimates in future studies. The accuracy of measuring the degree of κ-CN hydrolysis has implications on the precision of the data in relation to its partitioning (% distribution between the curd and whey) which is essential for improving whey quality.
Keywords: bovine milk; caseinoglycomacropeptide; caseinomacropeptide; cheese; rennet; κ‐CN hydrolysis.
© 2023 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The author declares no conflict of interest.
Figures

Similar articles
-
Genetic variation and posttranslational modification of bovine κ-casein: effects on caseino-macropeptide release during renneting.J Dairy Sci. 2015 Feb;98(2):747-58. doi: 10.3168/jds.2014-8678. Epub 2014 Dec 12. J Dairy Sci. 2015. PMID: 25497797
-
Milk protein fractions strongly affect the patterns of coagulation, curd firming, and syneresis.J Dairy Sci. 2019 Apr;102(4):2903-2917. doi: 10.3168/jds.2018-15524. Epub 2019 Feb 14. J Dairy Sci. 2019. PMID: 30772026
-
Effects of the detailed protein composition of milk on curd yield and composition measured by model micro-cheese curd making of individual milk samples.J Dairy Sci. 2019 Sep;102(9):7863-7873. doi: 10.3168/jds.2018-15743. Epub 2019 Jul 17. J Dairy Sci. 2019. PMID: 31326163
-
Micelle stability: kappa-casein structure and function.J Dairy Sci. 1998 Nov;81(11):3004-12. doi: 10.3168/jds.S0022-0302(98)75864-3. J Dairy Sci. 1998. PMID: 9839241 Review.
-
Plant-derived rennet: research progress, novel strategies for their isolation, identification, mechanism, bioactive peptide generation, and application in cheese manufacturing.Crit Rev Food Sci Nutr. 2025;65(3):444-456. doi: 10.1080/10408398.2023.2275295. Epub 2023 Oct 30. Crit Rev Food Sci Nutr. 2025. PMID: 37902764 Review.
References
-
- Alais, C. , Mocquot, G. , Nitschmann, H. S. , & Zahler, P. (1953). Das Lab und seine Wirkung auf das Casein der Milch. VII. Über die Abspaltung von Nicht‐Protein‐Stickstoff (NPN) aus Casein durch Lab und ihre Beziehung zur Primärreaktion der Labgerinnung der Milch. Helvetica Chimica Acta, 36, 1955–1968. 10.1002/hlca.19530360735 - DOI
-
- Anema, S. G. (2020). The whey proteins in milk: Thermal denaturation, physical interactions, and effects on the functional properties of milk. In Boland M. & Singh H. (Eds.), Milk proteins, from expression to food (3rd ed., pp. 325–384). Elsevier. 10.1016/B978-0-12-815251-5.00009-8 - DOI
-
- Anema, S. G. , Lee, S. K. , & Klostermeyer, H. (2007). Effect of pH at heat treatment on the hydrolysis of κ‐casein and the gelation of skim milk by chymosin. LWT‐Food Science and Technology, 40, 99–106. 10.1016/j.lwt.2005.08.002 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

