Tissue distribution and pharmacokinetics of isoxanthohumol from hops in rodents
- PMID: 38455172
- PMCID: PMC10916623
- DOI: 10.1002/fsn3.3900
Tissue distribution and pharmacokinetics of isoxanthohumol from hops in rodents
Abstract
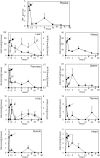
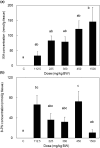
Vegetables and fruits contain prenylflavonoids with biological functions that might improve human health. The prenylflavonoid isoxanthohumol (IXA) and its derivative, 8-prenylnaringenin (8-PN), have beneficial activities, including anti-cancer effects and suppression of insulin resistance. However, their pharmacokinetic profile is unclear. Previous studies suggested flavonoids have low systemic availability and are excreted via the feces. Therefore, this study investigated the tissue distribution dynamics of high-purity IXA (>90%) from hops administered orally, either singly (50 mg/kg body weight [BW]) or daily for 14 days (30 mg/kg BW), to mice. High-pressure liquid chromatography demonstrated that IXA was absorbed rapidly after a single administration and reached plasma maximum concentration (C max) (3.95 ± 0.81 μmol/L) by 0.5 h. IXA was present at high levels in the liver compared with the kidney, pancreas, lung, skeletal muscle, spleen, thymus, and heart. The highest IXA level after 14 days of IXA ingestion was observed in the liver, followed by the kidney, thymus, spleen, lung, and brain. There was no significant difference in IXA accumulation in tissues between the single and multiple dose groups. Analyses of the livers of rats treated with different concentrations of IXA (112.5-1500 mg/kg BW) once a day for 28 days demonstrated that IXA accumulated dose-dependently with a correlation coefficient of .813. The accumulation of 8-PN was dependent on the intake period but not the intake amount of IXA (correlation coefficient -.255). In summary, IXA and 8-PN were detected in tissues and organs up to 24 h after ingestion, suggesting that orally ingested IXA might have health benefits as a nutraceutical.
Keywords: 8‐prenylnaringenin; bioavailability; flavanone; hop flavonoid; phytoestrogen; prenylflavonoid.
© 2023 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine.J Nutr. 2006 Jul;136(7):1862-7. doi: 10.1093/jn/136.7.1862. J Nutr. 2006. PMID: 16772450 Clinical Trial.
-
8-Prenylnaringenin tissue distribution and pharmacokinetics in mice and its binding to human serum albumin and cellular uptake in human embryonic kidney cells.Food Sci Nutr. 2022 Jan 22;10(4):1070-1080. doi: 10.1002/fsn3.2733. eCollection 2022 Apr. Food Sci Nutr. 2022. PMID: 35432956 Free PMC article.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
-
Eubacterium limosum activates isoxanthohumol from hops (Humulus lupulus L.) into the potent phytoestrogen 8-prenylnaringenin in vitro and in rat intestine.J Nutr. 2008 Jul;138(7):1310-6. doi: 10.1093/jn/138.7.1310. J Nutr. 2008. PMID: 18567753
-
Xanthohumol and related prenylflavonoids from hops and beer: to your good health!Phytochemistry. 2004 May;65(10):1317-30. doi: 10.1016/j.phytochem.2004.04.025. Phytochemistry. 2004. PMID: 15231405 Review.
Cited by
-
Antioxidant Potential of Xanthohumol in Disease Prevention: Evidence from Human and Animal Studies.Antioxidants (Basel). 2024 Dec 19;13(12):1559. doi: 10.3390/antiox13121559. Antioxidants (Basel). 2024. PMID: 39765887 Free PMC article. Review.
References
-
- Alert, O. (2005). Guidance for industry estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER).
-
- Bai, H. H. , Xia, T. S. , Jiang, Y. P. , Xu, W. M. , Xu, P. C. , Wang, N. N. , Gou, X. J. , & Xin, H. L. (2022). Absorption, metabolism, and pharmacokinetic profile of xanthohumol in rats as determined via UPLC‐MS/MS. Biopharmaceutics and Drug Disposition, 43(1), 11–22. 10.1002/bdd.2306 - DOI - PubMed
-
- Bai, Y. , Peng, W. , Yang, C. , Zou, W. , Liu, M. , Wu, H. , Fan, L. , Li, P. , Zeng, X. , & Su, W. (2020). Pharmacokinetics and metabolism of Naringin and active metabolite Naringenin in rats, dogs, humans, and the differences between species. Frontiers in Pharmacology, 11, 364. 10.3389/fphar.2020.00364 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

