Dietary therapy of the herbal porridge improves the symptoms of functional dyspepsia: A randomized, double-blind, placebo-controlled, clinical trial
- PMID: 38455174
- PMCID: PMC10916651
- DOI: 10.1002/fsn3.3911
Dietary therapy of the herbal porridge improves the symptoms of functional dyspepsia: A randomized, double-blind, placebo-controlled, clinical trial
Abstract
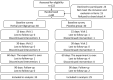
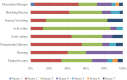
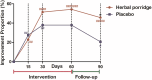
This study (ISRCTN17174559) aimed to explore the efficacy and safety of a kind of herbal porridge (Hou Gu Mi Xi) on the clinical symptoms of functional dyspepsia (FD). This was a single-center, single-dose, prospective, double-blind, randomized controlled trial involving 64 participants with FD (35 cases and 29 controls) for 2 months of intervention and 1 month of follow-up. The 7-point Global Overall Symptom Scale (GOSS), 36-Item Short Form Survey (SF-36), and other indicators were assessed at baseline (day 0), at days 15, 30, and 60 of treatment, and at follow-up 1 month after the end of the intervention. Many participants with FD achieved remission of their epigastric symptoms at follow-up on the 90th day after treatment with herbal porridge compared to the placebo group (45.71% vs. 20.69%, p = .036). Furthermore, herbal porridge appeared to be effective in improving the quality of life of participants with FD, which was reflected in the rising SF-36 scores for physical role, bodily pain, emotional role, and mental health. Although adverse events were reported, there was no overall difference in the number of adverse events between the two groups (p = .578). Herbal porridge is another effective and safe method for improving the symptoms and quality of life in patients with FD.
Keywords: dietary therapy; epigastric symptoms; functional dyspepsia; herbal porridge; randomized controlled trial; traditional Chinese medicine.
© 2024 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Epigastric pain syndrome: What can traditional Chinese medicine do? A randomized controlled trial of Biling Weitong Granules.World J Gastroenterol. 2020 Jul 28;26(28):4170-4181. doi: 10.3748/wjg.v26.i28.4170. World J Gastroenterol. 2020. PMID: 32821078 Free PMC article. Clinical Trial.
-
Banha-sasim-tang as an herbal formula for the treatment of functional dyspepsia: a randomized, double-blind, placebo-controlled, two-center trial.Trials. 2010 Jul 30;11:83. doi: 10.1186/1745-6215-11-83. Trials. 2010. PMID: 20670451 Free PMC article. Clinical Trial.
-
Efficacy and safety of Xiangsha Liujunzi granules for functional dyspepsia: A multi-center randomized double-blind placebo-controlled clinical study.World J Gastroenterol. 2017 Aug 14;23(30):5589-5601. doi: 10.3748/wjg.v23.i30.5589. World J Gastroenterol. 2017. PMID: 28852318 Free PMC article. Clinical Trial.
-
Efficacy and safety of Zhishixiaopi decoction in functional dyspepsia: A meta-analysis of randomized controlled trials.PLoS One. 2024 May 29;19(5):e0301686. doi: 10.1371/journal.pone.0301686. eCollection 2024. PLoS One. 2024. PMID: 38809916 Free PMC article.
-
Efficacy and Safety of STW 5-II for Functional Dyspepsia Treatment: A Patient Data-Based Meta-Analysis.Digestion. 2024;105(3):166-174. doi: 10.1159/000535672. Epub 2024 Jan 19. Digestion. 2024. PMID: 38246134 Free PMC article.
References
-
- Chan, A. W. , Tetzlaff, J. M. , Altman, D. G. , Laupacis, A. , Gotzsche, P. C. , Krleza‐Jeric, K. , Hróbjartsson, A. , Mann, H. , Dickersin, K. , Berlin, J. A. , Doré, C. J. , Parulekar, W. R. , Summerskill, W. S. , Groves, T. , Schulz, K. F. , Sox, H. C. , Rockhold, F. W. , Rennie, D. , & Moher, D. (2013). SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Annals of Internal Medicine, 158(3), 200–207. 10.7326/0003-4819-158-3-201302050-00583 - DOI - PMC - PubMed
-
- Chen, X. , Nie, H. , Liu, W. , Zhou, X. , Nie, J. , Xie, B. , Chen, D. , Jiang, Y. , Zhang, K. , Fu, Y. , Yang, D. , Xiong, Y. , Zhao, Z. , Sun, X. , & Zhu, W. (2018). Efficacy and safety of Hou Gu mi xi on spleen qi deficiency in patients with nonorganic gastrointestinal disorders: Protocol for a multicenter, randomized, placebo‐controlled trial. Evidence‐based Complementary and Alternative Medicine, 2018, 1980491. 10.1155/2018/1980491 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

