Regulatory effects of mangiferin on LPS-induced inflammatory responses and intestinal flora imbalance during sepsis
- PMID: 38455195
- PMCID: PMC10916552
- DOI: 10.1002/fsn3.3907
Regulatory effects of mangiferin on LPS-induced inflammatory responses and intestinal flora imbalance during sepsis
Abstract
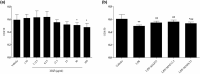
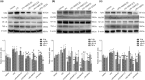
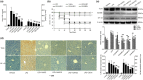
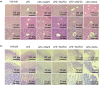
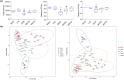
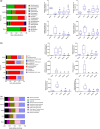
Studies suggest that mangiferin (MAF) has good therapeutic effects on chronic bronchitis and hepatitis. Also, it is one of the antiviral ingredients in Anemarrhena asphodeloides Bunge. However, its effect on the LPS-induced inflammation and intestinal flora during sepsis remains unclear yet. In the present study, LPS-stimulated inflammation RAW264.7 cells and LPS-induced sepsis mice were used to evaluate the efficacy of MAF in vitro and in vivo. 16S rDNA sequencing was performed to analyze the characteristics of intestinal flora of the sepsis mice. It has been demonstrated that MAF (12.5 and 25 μg/mL) significantly inhibited protein expressions of TLR4, MyD88, NF-κB, and TNF-α in the LPS-treated cells and reduced the supernatant TNF-α and IL-6 levels. In vivo, MAF (20 mg/kg) markedly protected the sepsis mice and reduced the serum TNF-α and IL-6 levels. Also, MAF significantly downregulated the protein expressions of TLR4, NF-κB, and MyD88 in the livers. Importantly, MAF significantly attenuated the pathological injuries of the livers and small intestines. Further, MAF significantly increased proportion of Bacteroidota and decreased the proportions of Firmicutes, Desulfobacterota, Actinobacteriota, and Proteobacteria at phylum level, and it markedly reduced the proportions of Escherichia-Shigella, Pseudoalteromonas, Staphylococcus at genus level. Moreover, MAF affects some metabolism-related pathways such as citrate cycle (TCA cycle), lipoic acid metabolism, oxidative phosphorylation, bacterial chemotaxis, fatty acid biosynthesis, and peptidoglycan biosynthesis of the intestinal flora. Thus, it can be concluded that MAF as a treatment reduces the inflammatory responses in vitro and in vivo by inhibiting the TLR4/ MyD88/NF-κB pathway, and corrects intestinal flora imbalance during sepsis to some degree.
Keywords: TLR4/myD88/NF‐κB; inflammation; intestinal flora; lipopolysaccharide; mangiferin.
© 2023 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
All the authors declared no conflict of interest in this study.
Figures
Similar articles
-
Electroacupuncture at Zusanli Alleviates Sepsis by Regulating the TLR4-MyD88-NF-Kappa B Pathway and Diversity of Intestinal Flora.Evid Based Complement Alternat Med. 2022 Jun 10;2022:6706622. doi: 10.1155/2022/6706622. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35722155 Free PMC article.
-
The role of TLR4/MyD88/NF-κB in the protective effect of ulinastatin on the intestinal mucosal barrier in mice with sepsis.BMC Anesthesiol. 2023 Dec 15;23(1):414. doi: 10.1186/s12871-023-02374-9. BMC Anesthesiol. 2023. PMID: 38102579 Free PMC article.
-
Ganluyin ameliorates DSS-induced ulcerative colitis by inhibiting the enteric-origin LPS/TLR4/NF-κB pathway.J Ethnopharmacol. 2022 May 10;289:115001. doi: 10.1016/j.jep.2022.115001. Epub 2022 Jan 24. J Ethnopharmacol. 2022. PMID: 35085745
-
Mangiferin ameliorates colitis by inhibiting IRAK1 phosphorylation in NF-κB and MAPK pathways.Eur J Pharmacol. 2014 Oct 5;740:652-61. doi: 10.1016/j.ejphar.2014.06.013. Epub 2014 Jun 24. Eur J Pharmacol. 2014. PMID: 24972244
-
Dihydrotanshinone exhibits an anti-inflammatory effect in vitro and in vivo through blocking TLR4 dimerization.Pharmacol Res. 2019 Apr;142:102-114. doi: 10.1016/j.phrs.2019.02.017. Epub 2019 Feb 19. Pharmacol Res. 2019. PMID: 30794925
Cited by
-
Warm and humid environment induces gut microbiota dysbiosis and bacterial translocation leading to inflammatory state and promotes proliferation and biofilm formation of certain bacteria, potentially causing sticky stool.BMC Microbiol. 2025 Jan 16;25(1):24. doi: 10.1186/s12866-024-03730-6. BMC Microbiol. 2025. PMID: 39819481 Free PMC article.
-
A Narrative Review of Protective Effects of Natural Compounds Against Lipopolysaccharide-Induced Injuries.Food Sci Nutr. 2025 Jan 31;13(2):e70026. doi: 10.1002/fsn3.70026. eCollection 2025 Feb. Food Sci Nutr. 2025. PMID: 39898124 Free PMC article. Review.
-
Altered cognitive function in obese patients: relationship to gut flora.Mol Cell Biochem. 2025 Jun;480(6):3553-3567. doi: 10.1007/s11010-024-05201-y. Epub 2025 Feb 12. Mol Cell Biochem. 2025. PMID: 39937394 Free PMC article. Review.
-
Evaluation of the protective role of resveratrol on LPS-induced septic intestinal barrier function via TLR4/MyD88/NF-κB signaling pathways.Sci Rep. 2025 Jan 4;15(1):828. doi: 10.1038/s41598-025-85148-2. Sci Rep. 2025. PMID: 39755761 Free PMC article.
-
Lacticaseibacillus rhamnosus HF01 postbiotics reprogram gut microbial tryptophan metabolism to coordinate enterohepatic barrier-insulin signaling axis.Curr Res Food Sci. 2025 Jun 10;11:101111. doi: 10.1016/j.crfs.2025.101111. eCollection 2025. Curr Res Food Sci. 2025. PMID: 40585863 Free PMC article.
References
-
- Cao, C. , Chai, Y. , Shou, S. , Wang, J. , Huang, Y. , & Ma, T. (2018). Toll‐like receptor 4 deficiency increases resistance in sepsis‐induced immune dysfunction. International Immunopharmacology, 54, 169–176. - PubMed
-
- Cao, C. , Yin, C. , Shou, S. , Wang, J. , Yu, L. , Li, X. , & Chai, Y. (2018). Ulinastatin protects against LPS‐induced acute lung injury by attenuating TLR4/NF‐κB pathway activation and reducing inflammatory mediators. Shock, 50, 595–605. - PubMed
LinkOut - more resources
Full Text Sources

