Molecular mechanisms underlying sarcopenia in heart failure
- PMID: 38455513
- PMCID: PMC10919908
- DOI: 10.20517/jca.2023.40
Molecular mechanisms underlying sarcopenia in heart failure
Abstract
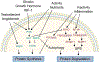
The loss of skeletal muscle, also known as sarcopenia, is an aging-associated muscle disorder that is disproportionately present in heart failure (HF) patients. HF patients with sarcopenia have poor outcomes compared to the overall HF patient population. The prevalence of sarcopenia in HF is only expected to grow as the global population ages, and novel treatment strategies are needed to improve outcomes in this cohort. Multiple mechanistic pathways have emerged that may explain the increased prevalence of sarcopenia in the HF population, and a better understanding of these pathways may lead to the development of therapies to prevent muscle loss. This review article aims to explore the molecular mechanisms linking sarcopenia and HF, and to discuss treatment strategies aimed at addressing such molecular signals.
Keywords: Heart failure; inflammation; mitochondria; proteostasis; sarcopenia; skeletal muscle.
Conflict of interest statement
DECLARATIONS Conflicts of interest The author declared that there are no conflicts of interest.
Figures

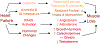


Similar articles
-
Exercise Capacity Is Improved by Levosimendan in Heart Failure and Sarcopenia via Alleviation of Apoptosis of Skeletal Muscle.Front Physiol. 2022 Jan 20;12:786895. doi: 10.3389/fphys.2021.786895. eCollection 2021. Front Physiol. 2022. PMID: 35126176 Free PMC article.
-
Exercise as a Therapeutic Strategy for Sarcopenia in Heart Failure: Insights into Underlying Mechanisms.Cells. 2020 Oct 13;9(10):2284. doi: 10.3390/cells9102284. Cells. 2020. PMID: 33066240 Free PMC article. Review.
-
Clinical impact of compound sarcopenia in hospitalized older adult patients with heart failure.J Am Geriatr Soc. 2021 Jul;69(7):1815-1825. doi: 10.1111/jgs.17108. Epub 2021 Mar 18. J Am Geriatr Soc. 2021. PMID: 33735939 Free PMC article.
-
The Role of Sarcopenia in Heart Failure with Depression.Rev Cardiovasc Med. 2022 Sep 5;23(9):296. doi: 10.31083/j.rcm2309296. eCollection 2022 Sep. Rev Cardiovasc Med. 2022. PMID: 39077715 Free PMC article. Review.
-
Age-Related Dysfunction in Proteostasis and Cellular Quality Control in the Development of Sarcopenia.Cells. 2023 Jan 7;12(2):249. doi: 10.3390/cells12020249. Cells. 2023. PMID: 36672183 Free PMC article. Review.
Cited by
-
Cancerous Conditions Accelerate the Aging of Skeletal Muscle via Mitochondrial DNA Damage.Int J Mol Sci. 2024 Jun 27;25(13):7060. doi: 10.3390/ijms25137060. Int J Mol Sci. 2024. PMID: 39000167 Free PMC article.
References
-
- Becher PM, Lund LH, Coats AJS, Savarese G. An update on global epidemiology in heart failure. Eur Heart J 2022;43:3005–7. - PubMed
-
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the american heart association. Circulation 2023;147:e93–621. - PubMed
-
- Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol 1993;22:6A–13A. - PubMed
-
- Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 2023;118:3272–87. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
