A Case of Long-Term Survival with Recurrent Liver Metastases from Gastric Cancer Treated with Nivolumab
- PMID: 38455714
- PMCID: PMC10919909
- DOI: 10.1159/000537779
A Case of Long-Term Survival with Recurrent Liver Metastases from Gastric Cancer Treated with Nivolumab
Abstract
Introduction: Improvements in overall survival from advanced gastric cancer have recently been reported with nivolumab. However, few reports have described long-term survival after discontinuing treatment.
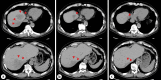
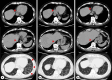
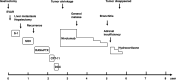
Case presentation: A 67-year-old man diagnosed with advanced gastric cancer and abdominal aortic aneurysm initially underwent distal gastrectomy with D2 dissection. Histological examination revealed tub2 and T2N1M0 stage IIA. One month later, endovascular aneurysm repair was performed. Six weeks after gastrectomy, adjuvant chemotherapy with S-1 was started. Six months later, liver metastases were identified and liver segments S1 and S7 were resected. S-1 and oxaliplatin were added postoperatively, but multiple liver metastases recurred. Paclitaxel and ramucirumab, irinotecan, and docetaxel were administered. Liver metastases showed a temporary reduction in size, then enlarged again. Nivolumab was therefore administered and the liver metastases showed a significant reduction in size. The interval between doses gradually increased due to persistent general fatigue. At 28 months after starting nivolumab therapy, bronchitis and adrenal insufficiency appeared, so treatment was discontinued. As of 3.5 years after cessation of nivolumab immunotherapy, tumor regression continued to be maintained. The patient remains alive as of 8 years after recurrence of liver metastases.
Conclusion: We encountered a case in which the patient received nivolumab therapy for recurrent liver metastases from gastric cancer and survived long term after discontinuing treatment.
Keywords: Gastric cancer; Liver metastases; Long survival; Nivolumab.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
Similar articles
-
Conversion surgery for stage IV gastric cancer with a complete pathological response to nivolumab: a case report.World J Surg Oncol. 2020 Jul 21;18(1):179. doi: 10.1186/s12957-020-01954-0. World J Surg Oncol. 2020. PMID: 32693806 Free PMC article.
-
Successful R0 resection after chemotherapy, including nivolumab, for gastric cancer with liver metastases: three case reports.Surg Case Rep. 2024 Jun 5;10(1):138. doi: 10.1186/s40792-024-01929-3. Surg Case Rep. 2024. PMID: 38837046 Free PMC article.
-
Successful treatment of liver metastases arising from early gastric cancer achieved clinical complete response by nivolumab.Surg Case Rep. 2018 Jul 5;4(1):71. doi: 10.1186/s40792-018-0479-3. Surg Case Rep. 2018. PMID: 29978335 Free PMC article.
-
[A Case of Neuroendocrine Carcinoma of the Stomach Treated with TAS-102].Gan To Kagaku Ryoho. 2022 Oct;49(10):1109-1111. Gan To Kagaku Ryoho. 2022. PMID: 36281604 Review. Japanese.
-
Liver metastases from gastric carcinoma: A Case report and review of the literature.Curr Probl Cancer. 2017 May-Jun;41(3):222-230. doi: 10.1016/j.currproblcancer.2017.03.003. Epub 2017 Mar 24. Curr Probl Cancer. 2017. PMID: 28625333 Review.
References
-
- Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. . Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

