Dynamic interactions in the tumor niche: how the cross-talk between CAFs and the tumor microenvironment impacts resistance to therapy
- PMID: 38455762
- PMCID: PMC10918473
- DOI: 10.3389/fmolb.2024.1343523
Dynamic interactions in the tumor niche: how the cross-talk between CAFs and the tumor microenvironment impacts resistance to therapy
Abstract
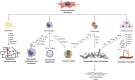
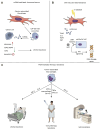
The tumor microenvironment (TME) is a complex ecosystem of cells, signaling molecules, and extracellular matrix components that profoundly influence cancer progression. Among the key players in the TME, cancer-associated fibroblasts (CAFs) have gained increasing attention for their diverse and influential roles. CAFs are activated fibroblasts found abundantly within the TME of various cancer types. CAFs contribute significantly to tumor progression by promoting angiogenesis, remodeling the extracellular matrix, and modulating immune cell infiltration. In order to influence the microenvironment, CAFs engage in cross-talk with immune cells, cancer cells, and other stromal components through paracrine signaling and direct cell-cell interactions. This cross-talk can result in immunosuppression, tumor cell proliferation, and epithelial-mesenchymal transition, contributing to disease progression. Emerging evidence suggests that CAFs play a crucial role in therapy resistance, including resistance to chemotherapy and radiotherapy. CAFs can modulate the tumor response to treatment by secreting factors that promote drug efflux, enhance DNA repair mechanisms, and suppress apoptosis pathways. This paper aims to understand the multifaceted functions of CAFs within the TME, discusses cross-talk between CAFs with other TME cells, and sheds light on the contibution of CAFs to therapy resistance. Targeting CAFs or disrupting their cross-talk with other cells holds promise for overcoming drug resistance and improving the treatment efficacy of various cancer types.
Keywords: cancer; cancer-associated fibroblasts; cross-talk; radiotherapy; therapy resistance; tumor-microenvironment.
Copyright © 2024 Piwocka, Piotrowski, Suchorska and Kulcenty.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Exploring the multifaceted role of direct interaction between cancer cells and fibroblasts in cancer progression.Front Mol Biosci. 2024 May 28;11:1379971. doi: 10.3389/fmolb.2024.1379971. eCollection 2024. Front Mol Biosci. 2024. PMID: 38863965 Free PMC article. Review.
-
Immune checkpoint inhibitors as mediators for immunosuppression by cancer-associated fibroblasts: A comprehensive review.Front Immunol. 2022 Oct 5;13:996145. doi: 10.3389/fimmu.2022.996145. eCollection 2022. Front Immunol. 2022. PMID: 36275750 Free PMC article. Review.
-
Cancer-associated fibroblasts and the tumor microenvironment in non-small cell lung cancer.Expert Rev Anticancer Ther. 2022 Feb;22(2):169-182. doi: 10.1080/14737140.2022.2019018. Epub 2022 Jan 2. Expert Rev Anticancer Ther. 2022. PMID: 34904919
-
Cancer-associated fibroblasts, tumor and radiotherapy: interactions in the tumor micro-environment.J Exp Clin Cancer Res. 2024 Dec 19;43(1):323. doi: 10.1186/s13046-024-03251-0. J Exp Clin Cancer Res. 2024. PMID: 39696386 Free PMC article. Review.
-
Cancer associated fibroblasts in cancer development and therapy.J Hematol Oncol. 2025 Mar 28;18(1):36. doi: 10.1186/s13045-025-01688-0. J Hematol Oncol. 2025. PMID: 40156055 Free PMC article. Review.
Cited by
-
Battlegrounds of treatment resistance: decoding the tumor microenvironment.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar 25. doi: 10.1007/s00210-025-04055-5. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40131387 Review.
-
Exosomal microRNAs: impact on cancer detection, treatment, and monitoring.Clin Transl Oncol. 2025 Jan;27(1):83-94. doi: 10.1007/s12094-024-03590-6. Epub 2024 Jul 6. Clin Transl Oncol. 2025. PMID: 38971914 Review.
-
Tumor-Associated Macrophages: Polarization, Immunoregulation, and Immunotherapy.Cells. 2025 May 19;14(10):741. doi: 10.3390/cells14100741. Cells. 2025. PMID: 40422244 Free PMC article. Review.
-
Contemporary Approaches to Immunotherapy of Solid Tumors.Cancers (Basel). 2024 Jun 19;16(12):2270. doi: 10.3390/cancers16122270. Cancers (Basel). 2024. PMID: 38927974 Free PMC article. Review.
-
Exploring the multifaceted role of direct interaction between cancer cells and fibroblasts in cancer progression.Front Mol Biosci. 2024 May 28;11:1379971. doi: 10.3389/fmolb.2024.1379971. eCollection 2024. Front Mol Biosci. 2024. PMID: 38863965 Free PMC article. Review.
References
-
- Aird W. C. (2007). Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circulation Res. 100 (2), 174–190. 10.1161/01.RES.0000255690.03436.AE - DOI - PubMed
-
- Arts R. J. W., Plantinga T. S., Tuit S., Ulas T., Heinhuis B., Tesselaar M., et al. (2016). Transcriptional and metabolic reprogramming induce an inflammatory phenotype in non-medullary thyroid carcinoma-induced macrophages. OncoImmunology 5 (12), e1229725. 10.1080/2162402X.2016.1229725 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

