Connecting single-nucleotide polymorphisms, glycosylation status, and interactions of plasma serine protease inhibitors
- PMID: 38455847
- PMCID: PMC10914678
- DOI: 10.1016/j.chempr.2022.11.018
Connecting single-nucleotide polymorphisms, glycosylation status, and interactions of plasma serine protease inhibitors
Abstract
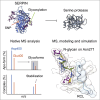
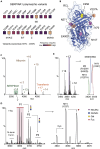
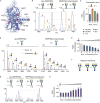
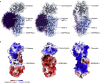
Understanding the combined impacts of genetic variances and post-translational modifications requires new approaches. Here, we delineate proteoforms of plasma serine protease inhibitors and relate specific proteoforms to their interactions in complexes through the use of native mass spectrometry (MS). First, we dissect the proteoform repertoire of an acute-phase plasma protein, serine protease inhibitor A1 (SERPINA1), resolving four SERPINA1 variants (M1V, M1A, M2, and M3) with common single-nucleotide polymorphisms (SNPs). Investigating the glycosylation status of these variants and their ability to form complexes with a serine protease, elastase, we find that fucosylation stabilizes the interaction of the SERPINA1 M1V variant through its core fucosylation on Asn271. In contrast, antennary fucosylation on Asn271 destabilizes SERPINA1-elastase interactions. We unveil the same opposing effects of core and antennary fucosylation on SERPINA3 interactions with chymotrypsin. Together, our native MS results highlight the modulating effects of fucosylation with different linkages on glycoprotein interactions.
Keywords: glycosylation; interaction; proteoform; serine protease inhibitors; single-nucleotide polymorphism.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Role of Serine Protease Inhibitors A1 and A3 in Ocular Pathologies.Invest Ophthalmol Vis Sci. 2024 Feb 1;65(2):16. doi: 10.1167/iovs.65.2.16. Invest Ophthalmol Vis Sci. 2024. PMID: 38324301 Free PMC article. Review.
-
The analysis of alpha-1-antitrypsin glycosylation with direct LC-MS/MS.Electrophoresis. 2018 Sep;39(18):2351-2361. doi: 10.1002/elps.201700426. Epub 2018 Feb 26. Electrophoresis. 2018. PMID: 29405331 Free PMC article.
-
Computational classification of core and outer fucosylation of N-glycoproteins in human plasma using collision-induced dissociation in mass spectrometry.Rapid Commun Mass Spectrom. 2020 Dec 15;34(23):e8917. doi: 10.1002/rcm.8917. Rapid Commun Mass Spectrom. 2020. PMID: 32754952
-
Machine Learning Classifies Core and Outer Fucosylation of N-Glycoproteins Using Mass Spectrometry.Sci Rep. 2020 Jan 15;10(1):318. doi: 10.1038/s41598-019-57274-1. Sci Rep. 2020. PMID: 31941975 Free PMC article.
-
Mass Spectrometric Studies of Apolipoprotein Proteoforms and Their Role in Lipid Metabolism and Type 2 Diabetes.Proteomes. 2017 Oct 15;5(4):27. doi: 10.3390/proteomes5040027. Proteomes. 2017. PMID: 29036931 Free PMC article. Review.
Cited by
-
Dissecting the structural heterogeneity of proteins by native mass spectrometry.Protein Sci. 2023 Apr;32(4):e4612. doi: 10.1002/pro.4612. Protein Sci. 2023. PMID: 36851867 Free PMC article. Review.
-
Identification of noval diagnostic biomarker for HFpEF based on proteomics and machine learning.Proteome Sci. 2025 Apr 8;23(1):3. doi: 10.1186/s12953-025-00242-7. Proteome Sci. 2025. PMID: 40200297 Free PMC article.
-
Tandem duplication of serpin genes yields functional variation and snake venom inhibitors.bioRxiv [Preprint]. 2025 Jan 10:2025.01.07.631777. doi: 10.1101/2025.01.07.631777. bioRxiv. 2025. PMID: 39829938 Free PMC article. Preprint.
-
Role of Serine Protease Inhibitors A1 and A3 in Ocular Pathologies.Invest Ophthalmol Vis Sci. 2024 Feb 1;65(2):16. doi: 10.1167/iovs.65.2.16. Invest Ophthalmol Vis Sci. 2024. PMID: 38324301 Free PMC article. Review.
-
The complete assembly of human LAT1-4F2hc complex provides insights into its regulation, function and localisation.Nat Commun. 2024 May 2;15(1):3711. doi: 10.1038/s41467-024-47948-4. Nat Commun. 2024. PMID: 38697966 Free PMC article.
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
