Crystal structure of the tetra-ethyl-ammonium salt of the non-steroidal anti-inflammatory drug nimesulide (polymorph II)
- PMID: 38456052
- PMCID: PMC10915663
- DOI: 10.1107/S2056989024001300
Crystal structure of the tetra-ethyl-ammonium salt of the non-steroidal anti-inflammatory drug nimesulide (polymorph II)
Abstract
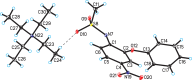
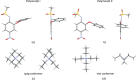
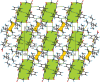
The crystal structure of the tetra-ethyl-ammonium salt of the non-steroidal anti-inflammatory drug nimesulide (polymorph II) (systematic name: tetra-ethyl-ammonium N-methane-sulfonyl-4-nitro-2-phen-oxy-anilinide), C8H20N+·C13H11N2O5S-, was determined using single-crystal X-ray diffraction. The title compound crystallizes in the monoclinic space group P21/c with one tetra-ethyl-ammonium cation and one nimesulide anion in the asymmetric unit. In the crystal, the ions are linked by C-H⋯N and C-H⋯O hydrogen bonds and C-H⋯π inter-actions. There are differences in the geometry of both the nimesulide anion and the tetra-ethyl-ammonium cation in polymorphs I [Rybczyńska & Sikorski (2023 ▸). Sci. Rep. 13, 17268] and II of the title compound.
Keywords: API; N-(4-nitro-2-phenoxyphenyl)methanesulfonamide; crystal structure; nimesulide; polymorphism; tetraethylammonium salt.
© Rybczyńska and Sikorski 2024.
Figures
References
-
- Banti, C. N., Papatriantafyllopoulou, C., Manoli, M., Tasiopoulos, A. J. & Hadjikakou, S. K. (2016). Inorg. Chem. 55, 8681–8696. - PubMed
-
- Bennett, A. & Villa, G. (2000). Expert Opin. Pharmacother. 1, 277–286. - PubMed
-
- Dupont, L., Pirotte, B., Masereel, B., Delarge, J. & Geczy, J. (1995). Acta Cryst. C51, 507–509.
LinkOut - more resources
Full Text Sources
