How the forebrain transitions to adulthood: developmental plasticity markers in a long-lived rodent reveal region diversity and the uniqueness of adolescence
- PMID: 38456144
- PMCID: PMC10917993
- DOI: 10.3389/fnins.2024.1365737
How the forebrain transitions to adulthood: developmental plasticity markers in a long-lived rodent reveal region diversity and the uniqueness of adolescence
Abstract
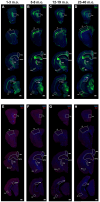
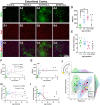
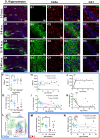
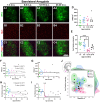
Maturation of the forebrain involves transitions from higher to lower levels of synaptic plasticity. The timecourse of these changes likely differs between regions, with the stabilization of some networks scaffolding the development of others. To gain better insight into neuroplasticity changes associated with maturation to adulthood, we examined the distribution of two molecular markers for developmental plasticity. We conducted the examination on male and female degus (Octodon degus), a rodent species with a relatively long developmental timecourse that offers a promising model for studying both development and age-related neuropathology. Immunofluorescent staining was used to measure perineuronal nets (PNNs), an extracellular matrix structure that emerges during the closure of critical plasticity periods, as well as microglia, resident immune cells that play a crucial role in synapse remodeling during development. PNNs (putatively restricting plasticity) were found to be higher in non-juvenile (>3 month) degus, while levels of microglia (putatively mediating plasticity) decreased across ages more gradually, and with varying timecourses between regions. Degus also showed notable variation in PNN levels between cortical layers and hippocampal subdivisions that have not been previously reported in other species. These results offer a glimpse into neuroplasticity changes occurring during degu maturation and highlight adolescence as a unique phase of neuroplasticity, in which PNNs have been established but microglia remain relatively high.
Keywords: adolescence; degu (Octodon degus); microglia; perineuronal net (PNN); plasticity.
Copyright © 2024 Garduño, Hanni, Hays, Cogram, Insel and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






Similar articles
-
Cognitively impaired aged Octodon degus recapitulate major neuropathological features of sporadic Alzheimer's disease.Acta Neuropathol Commun. 2022 Dec 19;10(1):182. doi: 10.1186/s40478-022-01481-x. Acta Neuropathol Commun. 2022. PMID: 36529803 Free PMC article.
-
Region-specific Effects of Maternal Separation on Perineuronal Net and Parvalbumin-expressing Interneuron Formation in Male and Female Rats.Neuroscience. 2020 Jan 21;428:23-37. doi: 10.1016/j.neuroscience.2019.12.010. Epub 2019 Dec 27. Neuroscience. 2020. PMID: 31887358
-
Influences of age and pubertal status on number and intensity of perineuronal nets in the rat medial prefrontal cortex.Brain Struct Funct. 2020 Nov;225(8):2495-2507. doi: 10.1007/s00429-020-02137-z. Epub 2020 Sep 10. Brain Struct Funct. 2020. PMID: 32914251 Free PMC article.
-
Perineuronal Nets: Plasticity, Protection, and Therapeutic Potential.Trends Neurosci. 2019 Jul;42(7):458-470. doi: 10.1016/j.tins.2019.04.003. Epub 2019 Jun 4. Trends Neurosci. 2019. PMID: 31174916 Review.
-
Perineuronal nets: Cruise from a honeycomb to the safety nets.Brain Res Bull. 2022 Nov;190:179-194. doi: 10.1016/j.brainresbull.2022.10.004. Epub 2022 Oct 7. Brain Res Bull. 2022. PMID: 36216273 Review.
Cited by
-
Octodon degus laboratory colony management principles and methods for behavioral analysis for Alzheimer's disease neuroscience research.Front Aging Neurosci. 2025 Jan 20;16:1517416. doi: 10.3389/fnagi.2024.1517416. eCollection 2024. Front Aging Neurosci. 2025. PMID: 39902280 Free PMC article.
References
-
- Andersen P., Morris R., Amaral D., Bliss T., O'Keefe J. (eds) (2006). The Hippocampus Book. Oxford: Oxford University Press. 10.1093/acprof:oso/9780195100273.001.0001 - DOI

