Influences on clinical trial participation: Enhancing recruitment through a gender lens - A scoping review
- PMID: 38456181
- PMCID: PMC10918492
- DOI: 10.1016/j.conctc.2024.101283
Influences on clinical trial participation: Enhancing recruitment through a gender lens - A scoping review
Abstract
Background: Suboptimal clinical trial recruitment contributes to research waste. Evidence suggests there may be gender-based differences in willingness to participate in clinical research. Identifying gender-based differences impacting the willingness of trial participation may assist trial recruitment.
Objectives: To examine factors that influence the willingness of men and women to participate in clinical trials and to identify modifiable factors that may be targeted to optimise trial participation.
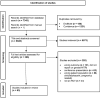
Material and methods: Electronic databases were searched with key words relating to 'gender', 'willingness to participate' and 'trial'. Included studies were English language and reported gender-based differences in willingness to participate in clinical trials, or factors that influence a single gender to participate in clinical trials. Studies were excluded if they described the demographic factors of trial participants or if the majority of participants were pregnant. Extracted data were coded, categorized, analysed thematically and interpreted using Arksey and O'Malley's framework.
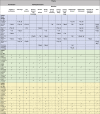
Results: Sixty-three studies were included. Two main themes were identified: trial characteristics and participant characteristics. A number of gender-based differences moderating willingness to participate were observed although only one, 'concern for self' was found to influence actual trial participation rates between genders.
Conclusion: The relationship between factors influencing willingness to participate in clinical trials is complex. The influence of gender on willingness to participate, while important, may be moderated by other factors including socioeconomic status, ethnicity and health condition. Exploring factors that influence willingness to participate specific to a study cohort likely offers the most promise to optimise trial recruitment of that cohort.
Keywords: Gender; Participation; Recruitment; Scoping review; Trial; Willingness.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Impact of gender on the willingness to participate in clinical trials and undergo related procedures in individuals from an Alzheimer's prevention research cohort.Alzheimers Res Ther. 2024 Dec 19;16(1):263. doi: 10.1186/s13195-024-01626-1. Alzheimers Res Ther. 2024. PMID: 39702338 Free PMC article.
-
Factors influencing the participation of pregnant and lactating women in clinical trials: A mixed-methods systematic review.PLoS Med. 2024 May 30;21(5):e1004405. doi: 10.1371/journal.pmed.1004405. eCollection 2024 May. PLoS Med. 2024. PMID: 38814991 Free PMC article.
-
Involving South Asian patients in clinical trials.Health Technol Assess. 2004 Oct;8(42):iii, 1-109. doi: 10.3310/hta8420. Health Technol Assess. 2004. PMID: 15488164 Review.
-
Understanding willingness and barriers to participate in clinical trials during pregnancy and lactation: findings from a US study.BMC Pregnancy Childbirth. 2024 Jul 26;24(1):504. doi: 10.1186/s12884-024-06710-w. BMC Pregnancy Childbirth. 2024. PMID: 39060985 Free PMC article.
-
Public attitudes regarding willingness to participate in medical research studies.J Health Soc Policy. 2000;12(2):23-43. doi: 10.1300/J045v12n02_02. J Health Soc Policy. 2000. PMID: 11184441
References
-
- Bondemark L., Ruf S. Randomized controlled trial: the gold standard or an unobtainable fallacy? Eur. J. Orthod. 2015;37(5):457–461. - PubMed
-
- Mouw T.J., Hong S.W., Sarwar S., Fondaw A.E., Walling A.D., Al-Kasspooles M., et al. Discontinuation of surgical versus nonsurgical clinical trials: an analysis of 88,498 trials. J. Surg. Res. 2018;227:151–157. - PubMed
Publication types
LinkOut - more resources
Full Text Sources