Thrombocytopenia Independently Leads to Changes in Monocyte Immune Function
- PMID: 38456277
- PMCID: PMC11069346
- DOI: 10.1161/CIRCRESAHA.123.323662
Thrombocytopenia Independently Leads to Changes in Monocyte Immune Function
Abstract
Background: While platelets have well-studied hemostatic functions, platelets are immune cells that circulate at the interface between the vascular wall and white blood cells. The physiological implications of these constant transient interactions are poorly understood. Activated platelets induce and amplify immune responses, but platelets may also maintain immune homeostasis in healthy conditions, including maintaining vascular integrity and T helper cell differentiation, meaning that platelets are central to both immune responses and immune quiescence. Clinical data have shown an association between low platelet counts (thrombocytopenia) and immune dysfunction in patients with sepsis and extracorporeal membrane oxygenation, further implicating platelets as more holistic immune regulators, but studies of platelet immune functions in nondisease contexts have had limited study.
Methods: We used in vivo models of thrombocytopenia and in vitro models of platelet and monocyte interactions, as well as RNA-seq and ATAC-seq (assay for transposase-accessible chromatin with sequencing), to mechanistically determine how resting platelet and monocyte interactions immune program monocytes.
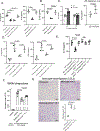
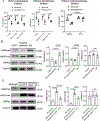
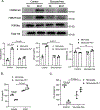
Results: Circulating platelets and monocytes interact in a CD47-dependent manner to regulate monocyte metabolism, histone methylation, and gene expression. Resting platelet-monocyte interactions limit TLR (toll-like receptor) signaling responses in healthy conditions in an innate immune training-like manner. In both human patients with sepsis and mouse sepsis models, thrombocytopenia exacerbated monocyte immune dysfunction, including increased cytokine production.
Conclusions: Thrombocytopenia immune programs monocytes in a manner that may lead to immune dysfunction in the context of sepsis. This is the first demonstration that sterile, endogenous cell interactions between resting platelets and monocytes regulate monocyte metabolism and pathogen responses, demonstrating platelets to be immune rheostats in both health and disease.
Keywords: acute coronary syndrome; chromatin assembly and disassembly; monocytes; platelet count; toll-like receptor agonists.
Conflict of interest statement
Figures





Update of
-
Thrombocytopenia Independently Leads to Monocyte Immune Dysfunction.bioRxiv [Preprint]. 2023 May 12:2023.05.10.540214. doi: 10.1101/2023.05.10.540214. bioRxiv. 2023. Update in: Circ Res. 2024 Apr 12;134(8):970-986. doi: 10.1161/CIRCRESAHA.123.323662. PMID: 37214993 Free PMC article. Updated. Preprint.
Comment in
-
Unveiling Platelets as Immune Regulatory Cells.Circ Res. 2024 Apr 12;134(8):987-989. doi: 10.1161/CIRCRESAHA.124.324167. Epub 2024 Apr 11. Circ Res. 2024. PMID: 38603477 No abstract available.
-
Microvascular and Macrovascular Disease: The S'mores of Peripheral Artery Disease.Circ Cardiovasc Interv. 2025 Oct;18(10):e015961. doi: 10.1161/CIRCINTERVENTIONS.125.015961. Epub 2025 Sep 22. Circ Cardiovasc Interv. 2025. PMID: 40977390 No abstract available.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

