Harnessing RNA Interference for Cholesterol Lowering: The Bench-to-Bedside Story of Inclisiran
- PMID: 38456415
- PMCID: PMC11010004
- DOI: 10.1161/JAHA.123.032031
Harnessing RNA Interference for Cholesterol Lowering: The Bench-to-Bedside Story of Inclisiran
Abstract
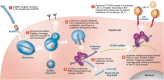
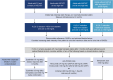
Lowering low-density lipoprotein cholesterol (LDL-C) is a cornerstone of reducing risk for atherosclerotic cardiovascular disease. Despite the approval of nonstatin therapies for LDL-C lowering over the past 2 decades, these medications are underused, and most patients are still not at guideline-recommended LDL-C goals. Barriers include poor adherence, clinical inertia, concern for side effects, cost, and complex prior authorization processes. With atherosclerotic cardiovascular disease-related mortality increasing globally, there remains a need for additional therapeutic options for lowering LDL-C as part of an atherosclerotic cardiovascular disease prevention strategy. Following the identification of PCSK9 (proprotein convertase subtilisin/kexin type 9) as a promising therapeutic target, inclisiran was developed using the natural process of RNA interference for robust, sustained prevention of hepatic PCSK9 synthesis. Twice-yearly maintenance subcutaneous inclisiran (following initial loading doses at Day 1 and Day 90) reduces circulating LDL-C levels by ≈50% versus placebo when added to maximally tolerated statins. Long-term safety and tolerability of inclisiran have been assessed, with studies underway to evaluate the effects of inclisiran on cardiovascular outcomes and to provide additional safety and effectiveness data. In 2021, <20 years after the discovery of PCSK9, inclisiran became the first RNA interference therapeutic approved in the United States for LDL-C lowering in patients with established atherosclerotic cardiovascular disease or familial hypercholesterolemia and has since been approved for use in patients with primary hyperlipidemia. This article reviews the journey of inclisiran from bench to bedside, including early development, the clinical trial program, key characteristics of inclisiran, and practical points for its use in the clinic.
Keywords: atherosclerotic cardiovascular disease; inclisiran; low‐density lipoprotein cholesterol; proprotein convertase subtilisin/kexin type 9; small interfering RNA.
Figures

References
-
- Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 - DOI - PubMed
-
- Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, et al. Low‐density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144 - DOI - PMC - PubMed
-
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625 - DOI - PMC - PubMed
-
- Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

