The clinical and genetic spectrum of inherited glycosylphosphatidylinositol deficiency disorders
- PMID: 38456468
- PMCID: PMC11292905
- DOI: 10.1093/brain/awae056
The clinical and genetic spectrum of inherited glycosylphosphatidylinositol deficiency disorders
Abstract
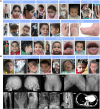
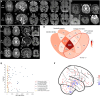
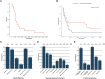
Inherited glycosylphosphatidylinositol deficiency disorders (IGDs) are a group of rare multisystem disorders arising from pathogenic variants in glycosylphosphatidylinositol anchor pathway (GPI-AP) genes. Despite associating 24 of at least 31 GPI-AP genes with human neurogenetic disease, prior reports are limited to single genes without consideration of the GPI-AP as a whole and with limited natural history data. In this multinational retrospective observational study, we systematically analyse the molecular spectrum, phenotypic characteristics and natural history of 83 individuals from 75 unique families with IGDs, including 70 newly reported individuals; the largest single cohort to date. Core clinical features were developmental delay or intellectual disability (DD/ID, 90%), seizures (83%), hypotonia (72%) and motor symptoms (64%). Prognostic and biologically significant neuroimaging features included cerebral atrophy (75%), cerebellar atrophy (60%), callosal anomalies (57%) and symmetric restricted diffusion of the central tegmental tracts (60%). Sixty-one individuals had multisystem involvement including gastrointestinal (66%), cardiac (19%) and renal (14%) anomalies. Though dysmorphic features were appreciated in 82%, no single dysmorphic feature had a prevalence >30%, indicating substantial phenotypic heterogeneity. Follow-up data were available for all individuals, 15 of whom were deceased at the time of writing. Median age at seizure onset was 6 months. Individuals with variants in synthesis stage genes of the GPI-AP exhibited a significantly shorter time to seizure onset than individuals with variants in transamidase and remodelling stage genes of the GPI-AP (P = 0.046). Forty individuals had intractable epilepsy. The majority of individuals experienced delayed or absent speech (95%), motor delay with non-ambulance (64%), and severe-to-profound DD/ID (59%). Individuals with a developmental epileptic encephalopathy (51%) were at greater risk of intractable epilepsy (P = 0.003), non-ambulance (P = 0.035), ongoing enteral feeds (P < 0.001) and cortical visual impairment (P = 0.007). Serial neuroimaging showed progressive cerebral volume loss in 87.5% and progressive cerebellar atrophy in 70.8%, indicating a neurodegenerative process. Genetic analyses identified 93 unique variants (106 total), including 22 novel variants. Exploratory analyses of genotype-phenotype correlations using unsupervised hierarchical clustering identified novel genotypic predictors of clinical phenotype and long-term outcome with meaningful implications for management. In summary, we expand both the mild and severe phenotypic extremities of the IGDs, provide insights into their neurological basis, and vitally, enable meaningful genetic counselling for affected individuals and their families.
Keywords: GPI; congenital disorders of glycosylation; developmental delay; epilepsy; neurodevelopmental disorder; neuroimaging.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
J.S. is supported by Cancer Research UK (City of London Centre Award SEBCATP-2022/100008) and University College London. F.M. is supported by University College London. P.S. and A.R. are supported by PNRR-MUR-M4C2 PE0000006 Research Program ‘MNESYS’: a multiscale, integrated approach to the study of the nervous system in health and disease. Their institute, IRCCS Istituto Giannina Gaslini, is a member EpiCARE. M.S.Z. is supported by the Egypt Science and Technology Developmental Fund (STDF—33650). W.J. has received personal consultancy fees from Roche Products Limited. A.C.J. is supported by a Senior Clinical Investigator Fellowship from the Research Foundation Flanders. A.C.E. is supported by a US National Institutes of Health grant (U54 NS115198). L.M. has received personal consultancy fees from Mendelian Ltd., a rare disease digital health company, outside of the submitted work. A.M. is supported by the UK Medical Research Council (MR/T007087/1), Great Ormond Street Hospital (GOSH) Charity (VS0122), Rosetrees Trust, Wellcome Trust, and Great Ormond Street Hospital National Institute for Health Research Biomedical Research Centre. Outside of the submitted work, A.M. has also received consulting fees from Rocket Pharmaceuticals; honorarium from Jazz Pharmaceuticals; support for attending conferences from Jazz Pharmaceuticals and the European Paediatric Neurology Society; fees for participating on boards for Biogen and Biocodex; serves unpaid roles on the International League Against Epilepsy Genetic Literacy Task Force and EpiCARE; and Chair of Junior Faculty for the Great Ormond Street Hospital National Institute for Health and Care Research Biomedical Research Centre. K.M. provides private medicolegal expertise on instruction by the court and has received personal consultancy fees from The Cromwell Hospital, HCA Healthcare UK, European Society of Paediatric Neuroradiology, Society of Pediatric Neuroimaging, and the UK Crown Court, outside of the submitted work.
On behalf of all authors, the corresponding authors assert that no financial relationships exist with any organizations that might have an interest in the submitted work and that no other relationships or activities exist that could appear to have influenced the submitted work.
Figures



References
-
- Nozaki M, Ohishi K, Yamada N, Kinoshita T, Nagy A, Takeda J. Developmental abnormalities of glycosylphosphatidylinositol-anchor-deficient embryos revealed by Cre/loxP system. Lab Invest. 1999;79:293–299. - PubMed
-
- Alfieri JA, Martin AD, Takeda J, Kondoh G, Myles DG, Primakoff P. Infertility in female mice with an oocyte-specific knockout of GPI-anchored proteins. J Cell Sci. 2003;116(Pt 11):2149–2155. - PubMed
-
- Fujita M, Kinoshita T. GPI-anchor remodeling: Potential functions of GPI-anchors in intracellular trafficking and membrane dynamics. Biochim Biophys Acta. 2012;1821:1050–1058. - PubMed

