α‑Phellandrene enhances the apoptosis of HT‑29 cells induced by 5‑fluorouracil by modulating the mitochondria‑dependent pathway
- PMID: 38456489
- PMCID: PMC10940876
- DOI: 10.3892/or.2024.8720
α‑Phellandrene enhances the apoptosis of HT‑29 cells induced by 5‑fluorouracil by modulating the mitochondria‑dependent pathway
Abstract
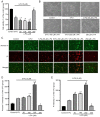
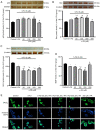
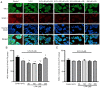
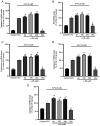
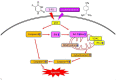
α‑Phellandrene (α‑PA), a natural constituent of herbs, inhibits cancer cell viability and proliferation. 5‑Fluorouracil (5‑FU) is a frequently utilized chemotherapeutic medicine for the treatment of colon cancer, which works by triggering cancer cell apoptosis. The present study examined how the combination of α‑PA and 5‑FU affects the suppression of human colon cancer cells by promoting apoptosis. The impact of this treatment on cell viability, apoptosis, and the expression levels of Bcl‑2 family members, caspase family members and mitochondria‑related molecules in HT‑29 cells was assessed by the MTT assay, immunocytochemistry, western blotting and quantitative PCR. The combination of 5‑FU and α‑PA had a synergistic inhibitory effect on cell viability, as determined by assessing the combination index value. Bax protein expression levels were higher in the 50, 100 or 250 µM α‑PA combined with 5‑FU groups compared with those in the 5‑FU alone group (P<0.05). By contrast, Bcl‑2 protein expression levels and mitochondrial membrane potential (MMP, ΔΨm) were lower in the 100 or 250 µM α‑PA combined with 5‑FU groups than those in the 5‑FU alone group (P<0.05). In addition, hexokinase‑2 (HK‑2) protein expression levels were lower in the 50, 100 or 250 µM α‑PA combined with 5‑FU groups than those in the 5‑FU alone group (P<0.05). Compared with 5‑FU alone, after HT‑29 cells were treated with 50, 100 or 250 µM α‑PA combined with 5‑FU, the mRNA expression levels of extrinsic‑induced apoptotic molecules, including caspase‑8 and Bid, were higher (P<0.05). Treatment with 50, 100 or 250 µM α‑PA combined with 5‑FU also increased the mRNA expression levels of cytochrome c, caspase‑9 and caspase‑3, regulating intrinsic apoptosis (P<0.05). These results showed that α‑PA and 5‑FU had a synergistic effect on reducing the viability of human colon cancer HT‑29 cells by inducing extrinsic and intrinsic apoptosis pathways. The mechanism by which apoptosis is induced may involve the intrinsic apoptosis pathway that activates the mitochondria‑dependent pathway, including regulating the expression levels of Bcl‑2 family members, including Bax, Bcl‑2 and Bid, regulating MMP and HK‑2 expression levels, and increasing the expression of caspase cascade molecules, including caspase‑9 and caspase‑3. In addition, it may involve the extrinsic apoptosis pathway that activates caspase‑8 and caspase‑3 leading to apoptosis.
Keywords: 5‑fluorouracil; Bcl‑2 family; HT‑29 cells; apoptosis; caspase family; mitochondria; α‑phellandrene.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Riyazi A, Hensel A, Bauer K, Geissler N, Schaaf S, Verspohl EJ. The effect of the volatile oil from ginger rhizomes (Zingiber officinale), its fractions and isolated compounds on the 5-HT3 receptor complex and the serotoninergic system of the rat ileum. Planta Med. 2007;73:355–362. doi: 10.1055/s-2007-967171. - DOI - PubMed
-
- Dimov M, Petkova Z, Stoyanova M, Dobreva K, Stoyanova A. Comparative chemical composition of dill fruits of different origins. Bulg J Agric Sci. 2020;26:696–700.
-
- Prasannakumar NR, Jyothi N, Saroja S, Lokesha AN. Insecticidal properties of Ocimum basilicum and Mentha piperita essential oils against South American Tomato moth, Phthorimaea absoluta (Meyrick) (Lepidoptera: Gelichiidae) Pestic Biochem Physiol. 2023;190:105329. doi: 10.1016/j.pestbp.2022.105329. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

