Age-related dysregulation of intestinal epithelium fucosylation is linked to an increased risk of colon cancer
- PMID: 38456503
- PMCID: PMC10972600
- DOI: 10.1172/jci.insight.167676
Age-related dysregulation of intestinal epithelium fucosylation is linked to an increased risk of colon cancer
Abstract
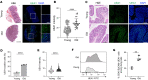
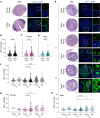
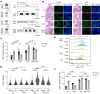
Colon cancer affects people of all ages. However, its frequency, as well as the related morbidity and mortality, are high among older adults. The complex physiological changes in the aging gut substantially limit the development of cancer therapies. Here, we identify a potentially unique intestinal microenvironment that is linked with an increased risk of colon cancer in older adults. Our findings show that aging markedly influenced persistent fucosylation of the apical surfaces of intestinal epithelial cells, which resulted in a favorable environment for tumor growth. Furthermore, our findings shed light on the importance of the host-commensal interaction, which facilitates the dysregulation of fucosylation and promotes tumor growth as people get older. We analyzed colonic microbial populations at the species level to find changes associated with aging that could contribute to the development of colon cancer. Analysis of single-cell RNA-sequencing data from previous publications identified distinct epithelial cell subtypes involved in dysregulated fucosylation in older adults. Overall, our study provides compelling evidence that excessive fucosylation is associated with the development of colon cancer, that age-related changes increase vulnerability to colon cancer, and that a dysbiosis in microbial diversity and metabolic changes in the homeostasis of older mice dysregulate fucosylation levels with age.
Keywords: Aging; Cellular senescence; Colorectal cancer; Drug therapy; Microbiology.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

