Cadherin-dependent adhesion is required for muscle stem cell niche anchorage and maintenance
- PMID: 38456551
- PMCID: PMC11057819
- DOI: 10.1242/dev.202387
Cadherin-dependent adhesion is required for muscle stem cell niche anchorage and maintenance
Abstract
Adhesion between stem cells and their niche provides stable anchorage and signaling cues to sustain properties such as quiescence. Skeletal muscle stem cells (MuSCs) adhere to an adjacent myofiber via cadherin-catenin complexes. Previous studies on N- and M-cadherin in MuSCs revealed that although N-cadherin is required for quiescence, they are collectively dispensable for MuSC niche localization and regenerative activity. Although additional cadherins are expressed at low levels, these findings raise the possibility that cadherins are unnecessary for MuSC anchorage to the niche. To address this question, we conditionally removed from MuSCs β- and γ-catenin, and, separately, αE- and αT-catenin, factors that are essential for cadherin-dependent adhesion. Catenin-deficient MuSCs break quiescence similarly to N-/M-cadherin-deficient MuSCs, but exit the niche and are depleted. Combined in vivo, ex vivo and single cell RNA-sequencing approaches reveal that MuSC attrition occurs via precocious differentiation, re-entry to the niche and fusion to myofibers. These findings indicate that cadherin-catenin-dependent adhesion is required for anchorage of MuSCs to their niche and for preservation of the stem cell compartment. Furthermore, separable cadherin-regulated functions govern niche localization, quiescence and MuSC maintenance.
Keywords: Cell adhesion; Cell signaling; Muscle stem cell; Quiescence; Stem cell niche.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
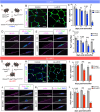
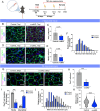

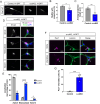

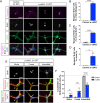
References
-
- Almada, A. E., Horwitz, N., Price, F. D., Gonzalez, A. E., Ko, M., Bolukbasi, O. V., Messemer, K. A., Chen, S., Sinha, M., Rubin, L. L.et al. (2021). FOS licenses early events in stem cell activation driving skeletal muscle regeneration. Cell Rep. 34, 108656. 10.1016/j.celrep.2020.108656 - DOI - PMC - PubMed
-
- Bates, D., Mächler, M., Bolker, B. and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. 10.18637/jss.v067.i01 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

