2-Aminophenanthroline Ligands Enable Mild, Undirected, Iridium-Catalyzed Borylation of Alkyl C-H Bonds
- PMID: 38456743
- PMCID: PMC11620480
- DOI: 10.1021/jacs.3c12981
2-Aminophenanthroline Ligands Enable Mild, Undirected, Iridium-Catalyzed Borylation of Alkyl C-H Bonds
Abstract
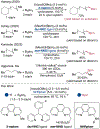
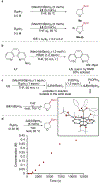
The catalytic, undirected borylation of alkyl C-H bonds typically occurs at high reaction temperatures or with excess substrate, or both, because of the low reactivity of alkyl C-H bonds. Here we report a new iridium system comprising 2-anilino-1,10-phenanthroline as the ligand that catalyzes the borylation of alkyl C-H bonds with little to no induction period and with high reaction rates. This superior activation and reactivity profile of 2-aminophenanthroline-ligated catalysts leads to broader reaction scope, including reactions of sensitive substrates, such as epoxides and glycosidic acetals, enhanced diastereoselectivity, and higher yields of borylated products. These catalysts also enable the borylation of alkanes, amines, and ethers at room temperature for the first time. Mechanistic studies imply that facile N-borylation occurs under the reaction conditions and that iridium complexes containing N-boryl aminophenanthrolines are competent precatalysts for the reaction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Wencel-Delord J; Glorius F C-H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem 2013, 5, 369–375. - PubMed
-
- Goldberg KI; Goldman AS Large-Scale Selective Functionalization of Alkanes. Acc. Chem. Res 2017, 50, 620–626. - PubMed
-
- Guillemard L; Kaplaneris N; Ackermann L; Johansson MJ Late-stage C-H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem 2021, 5, 522–545. - PubMed
-
- Hartwig JF Borylation and Silylation of C-H Bonds: A Platform for Diverse C-H Bond Functionalizations. Acc. Chem. Res 2012, 45, 864–873. - PubMed
-
- Yu IF; Wilson JW; Hartwig JF Transition-Metal-Catalyzed Silylation and Borylation of C-H Bonds for the Synthesis and Functionalization of Complex Molecules. Chem. Rev 2023, 123, 11619–11663. - PubMed

