Effect of 2-Week Naringin Supplementation on Neurogenesis and BDNF Levels in Ischemia-Reperfusion Model of Rats
- PMID: 38457013
- PMCID: PMC10924031
- DOI: 10.1007/s12017-023-08771-0
Effect of 2-Week Naringin Supplementation on Neurogenesis and BDNF Levels in Ischemia-Reperfusion Model of Rats
Abstract
Background: Ischemic stroke is the leading cause of mortality and disability worldwide with more than half of survivors living with serious neurological sequelae; thus, it has recently attracted a lot of attention in the field of medical study.
Purpose: The aim of this study was to determine the effect of naringin supplementation on neurogenesis and brain-derived neurotrophic factor (BDNF) levels in the brain in experimental brain ischemia-reperfusion.
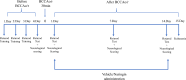
Study design: The research was carried out on 40 male Wistar-type rats (10-12 weeks old) obtained from the Experimental Animals Research and Application Center of Selçuk University. Experimental groups were as follows: (1) Control group, (2) Sham group, (3) Brain ischemia-reperfusion group, (4) Brain ischemia-reperfusion + vehicle group (administered for 14 days), and (5) Brain ischemia-reperfusion + Naringin group (100 mg/kg/day administered for 14 days).
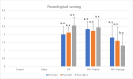
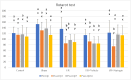
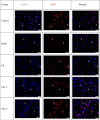
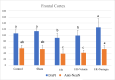
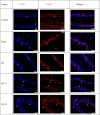
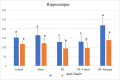
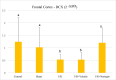
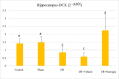
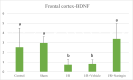
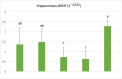
Methods: In the ischemia-reperfusion groups, global ischemia was performed in the brain by ligation of the right and left carotid arteries for 30 min. Naringin was administered to experimental animals by intragastric route for 14 days following reperfusion. The training phase of the rotarod test was started 4 days before ischemia-reperfusion, and the test phase together with neurological scoring was performed the day before and 1, 7, and 14 days after the operation. At the end of the experiment, animals were sacrificed, and then hippocampus and frontal cortex tissues were taken from the brain. Double cortin marker (DCX), neuronal nuclear antigen marker (NeuN), and BDNF were evaluated in hippocampus and frontal cortex tissues by Real-Time qPCR analysis and immunohistochemistry methods.
Results: While ischemia-reperfusion increased the neurological score values, DCX, NeuN, and BDNF levels decreased significantly after ischemia in the hippocampus and frontal cortex tissues. However, naringin supplementation restored the deterioration to a certain extent.
Conclusion: The results of the study show that 2 weeks of naringin supplementation may have protective effects on impaired neurogenesis and BDNF levels after brain ischemia and reperfusion in rats.
Keywords: BDNF; DCX; Flavonoid; Naringin; NeuN; Neurogenesis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Prabhakaran, S., Ruff, I., & Bernstein, R. A. (2015). Acute stroke intervention: A systematic review. JAMA,313, 1451–1462. - PubMed
-
- Marques, B. L., Carvalho, G. A., Freitas, E. M. M., Chiareli, R. A., Barbosa, T. G., Di Araujo, A. G. P., Nogueira, Y. L., Ribeiro, R. I., Parreira, R. C., Vieira, M. S., Resende, R. R., Gomez, R. S., Oliveira-Lima, O. C., & Pinto, M. C. X. (2019). The role of neurogenesis in neurorepair after ischemic stroke. Seminars in Cell & Developmental Biology,95, 98–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

