The generation of glioma organoids and the comparison of two culture methods
- PMID: 38457217
- PMCID: PMC10923046
- DOI: 10.1002/cam4.7081
The generation of glioma organoids and the comparison of two culture methods
Abstract
Background: The intra- and inter-tumoral heterogeneity of gliomas and the complex tumor microenvironment make accurate treatment of gliomas challenging. At present, research on gliomas mainly relies on cell lines, stem cell tumor spheres, and xenotransplantation models. The similarity between traditional tumor models and patients with glioma is very low.
Aims: In this study, we aimed to address the limitations of traditional tumor models by generating patient-derived glioma organoids using two methods that summarized the cell diversity, histological features, gene expression, and mutant profiles of their respective parent tumors and assess the feasibility of organoids for personalized treatment.
Materials and methods: We compared the organoids generated using two methods through growth analysis, immunohistological analysis, genetic testing, and the establishment of xenograft models.
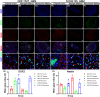
Results: Both types of organoids exhibited rapid infiltration when transplanted into the brains of adult immunodeficient mice. However, organoids formed using the microtumor method demonstrated more similar cellular characteristics and tissue structures to the parent tumors. Furthermore, the microtumor method allowed for faster culture times and more convenient operational procedures compared to the Matrigel method.
Discussion: Patient-derived glioma organoids, especially those generated through the microtumor method, present a promising avenue for personalized treatment strategies. Their capacity to faithfully mimic the cellular and molecular characteristics of gliomas provides a valuable platform for elucidating tumor biology and evaluating therapeutic modalities.
Conclusion: The success rates of the Matrigel and microtumor methods were 45.5% and 60.5%, respectively. The microtumor method had a higher success rate, shorter establishment time, more convenient passage and cryopreservation methods, better simulation of the cellular and histological characteristics of the parent tumor, and a high genetic guarantee.
Keywords: glioma; organoid; patient-derived orthotopic xenograft model; stem cell.
© 2024 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare there is no conflict of interest in the study.
Figures








References
-
- Fernandes C, Costa A, Osório L, et al. Current standards of care in glioblastoma therapy. In: De Vleeschouwer S, ed. Glioblastoma. Codon Publications; 2017. Chapter 11. - PubMed
-
- Wick W, Weller M, van den Bent M, et al. MGMT testing—the challenges for biomarker‐based glioma treatment. Nat Rev Neurol. 2014;10(7):372‐385. - PubMed
Publication types
MeSH terms
Grants and funding
- 82102770/National Natural Science Foundation of China
- 82173279/National Natural Science Foundation of China
- H2017064; H201621/General Research Project of Jiangsu Provincial Health Commission
- M2022050/General Research Project of Jiangsu Provincial Health Commission
- YCZYPT[2020]06-1/National Science and Technology Resource Sharing Service Platform Program
LinkOut - more resources
Full Text Sources

