Growing Awareness: Limited Testing and Screening Bias for Hepatitis Delta Virus in Utah 2000-2021
- PMID: 38457349
- PMCID: PMC11420768
- DOI: 10.1093/infdis/jiae023
Growing Awareness: Limited Testing and Screening Bias for Hepatitis Delta Virus in Utah 2000-2021
Abstract
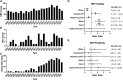
Background: This study assessed the epidemiology of hepatitis delta virus (HDV) within the University of Utah UHealth health care system (2000-2021).
Methods: Analysis of HDV/HBV testing, diagnostic codes, liver enzymes, and comorbidities was performed.
Results: Among the 1962 HBV patients, only 22.2% underwent HDV testing, revealing an 8.3% positivity rate for HDV coinfections. This study observed a consistent increase in HBV and HDV cases, with higher HDV detection rates linked to increased testing. Limited HDV testing and potential screening biases were evident.
Discussion: Improved HDV testing and surveillance are crucial for early detection and implementation of targeted therapies.
Keywords: hepatitis B virus; hepatitis D virus; hepatitis delta virus.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. M. L. W. and K. M. L. have current funding from Gilead Sciences, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Taylor JM, Purcell RH, Farci P. Fields virology. 6th ed. Vol 1. Philadelphia, PA: Lippincott Williams & Wilkins, 2013:2222–41.
-
- Farci P, Niro GA. Clinical features of hepatitis D. Semin Liver Dis 2012; 32:228–36. - PubMed
-
- Safaie P, Razeghi S, Rouster SD, Privitera I, Sherman KE. Hepatitis D diagnostics: utilization and testing in the United States. Virus Research 2018; 250:114–7. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

