MRD at the end of induction and EFS in T-cell lymphoblastic lymphoma: Children's Oncology Group trial AALL1231
- PMID: 38457359
- PMCID: PMC11143515
- DOI: 10.1182/blood.2023021184
MRD at the end of induction and EFS in T-cell lymphoblastic lymphoma: Children's Oncology Group trial AALL1231
Abstract
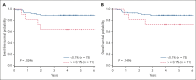
Defining prognostic variables in T-lymphoblastic lymphoma (T-LL) remains a challenge. AALL1231 was a Children's Oncology Group phase 3 clinical trial for newly diagnosed patients with T acute lymphoblastic leukemia or T-LL, randomizing children and young adults to a modified augmented Berlin-Frankfurt-Münster backbone to receive standard therapy (arm A) or with addition of bortezomib (arm B). Optional bone marrow samples to assess minimal residual disease (MRD) at the end of induction (EOI) were collected in T-LL analyzed to assess the correlation of MRD at the EOI to event-free survival (EFS). Eighty-six (41%) of the 209 patients with T-LL accrued to this trial submitted samples for MRD assessment. Patients with MRD <0.1% (n = 75) at EOI had a superior 4-year EFS vs those with MRD ≥0.1% (n = 11) (89.0% ± 4.4% vs 63.6% ± 17.2%; P = .025). Overall survival did not significantly differ between the 2 groups. Cox regression for EFS using arm A as a reference demonstrated that MRD EOI ≥0.1% was associated with a greater risk of inferior outcome (hazard ratio, 3.73; 95% confidence interval, 1.12-12.40; P = .032), which was independent of treatment arm assignment. Consideration to incorporate MRD at EOI into future trials will help establish its value in defining risk groups. CT# NCT02112916.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: S.P.H. received honoraria from Amgen; received consulting fees from Novartis; and owns common stock in Amgen. M.L.L. received consulting fees from MediSix Therapeutics. M.L.H. served on advisory boards for Novartis and Sobi. D.T.T. serves on advisory boards for Amgen, La Roche, Janssen, and Sobi. C.E.A. serves on advisory boards for Sobi and OPNA; and receives research support from Genentech. The remaining authors declare no competing financial interests.
Figures
Comment in
-
MRD: also for T-lymphoblastic lymphoma.Blood. 2024 May 16;143(20):2017-2019. doi: 10.1182/blood.2024024344. Blood. 2024. PMID: 38753352 No abstract available.
References
-
- Heerema NA, Sather HN, Sensel MG, et al. Frequency and clinical significance of cytogenetic abnormalities in pediatric T-lineage acute lymphoblastic leukemia: a report from the Children's Cancer Group. J Clin Oncol. 1998;16(4):1270–1278. - PubMed
-
- Lones MA, Heerema NA, Le Beau MM, et al. Chromosome abnormalities in advanced stage lymphoblastic lymphoma of children and adolescents: a report from CCG-E08. Cancer Genet Cytogenet. 2007;172(1):1–11. - PubMed
-
- Bonn BR, Rohde M, Zimmermann M, et al. Incidence and prognostic relevance of genetic variations in T-cell lymphoblastic lymphoma in childhood and adolescence. Blood. 2013;121(16):3153–3160. - PubMed
-
- Balbach ST, Makarova O, Bonn BR, et al. Proposal of a genetic classifier for risk group stratification in pediatric T-cell lymphoblastic lymphoma reveals differences from adult T-cell lymphoblastic leukemia. Leukemia. 2016;30(4):970–973. - PubMed
-
- Callens C, Baleydier F, Lengline E, et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J Clin Oncol. 2012;30(16):1966–1973. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical