New, simplified versus standard photodynamic therapy (PDT) regimen for superficial and nodular basal cell carcinoma (BCC): A single-blind, non-inferiority, randomised controlled multicentre study
- PMID: 38457386
- PMCID: PMC10923430
- DOI: 10.1371/journal.pone.0299718
New, simplified versus standard photodynamic therapy (PDT) regimen for superficial and nodular basal cell carcinoma (BCC): A single-blind, non-inferiority, randomised controlled multicentre study
Abstract
Background: Topical photodynamic therapy (PDT) is an approved and widely used treatment for low-risk basal cell carcinoma (BCC), comprising two sessions with an interval of 1 week. Simplification of the treatment course can be cost-effective, easier to organize, and cause less discomfort for the patients.
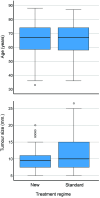
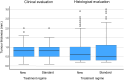
Methods and findings: We performed an investigator-initiated, single-blind, non-inferiority, randomized controlled multicentre study with the objective of investigating whether a simpler and more flexible PDT regimen was not >10% less effective than the standard double PDT in the treatment of primary, superficial, and nodular ≤2 mm-thick BCC and evaluate the cosmetic outcome. With a non-inferiority margin of 0.1 and an expected probability complete response of 0.85, 190 tumours were required in each group. Histologically verified BCCs from seven centres in Norway were randomly assigned (1:1) to either receive a new regimen of single PDT with one possible re-treatment of non-complete responding tumours, or the standard regimen. The primary endpoint was the number of tumours with complete response or treatment failure at 36 months of follow-up, assessed by investigators blinded to the treatment regimen. Intention-to-treat and per-protocol analyses were performed. The cosmetic outcome was recorded. The study was registered with ClinicalTrials.gov, NCT-01482104, and EudraCT, 2011-004797-28. A total of 402 BCCs in 246 patients were included; 209 tumours assigned to the new and 193 to the standard regimen. After 36 months, there were 61 treatment failures with the new and 34 failures with the standard regimen. Complete response rate was 69.5% in the new and 81.1% in the standard treatment group. The difference was 11.6% (upper 97.5% CI 20.3), i.e. > than the non-inferiority margin of 10%. Cosmetic outcomes were excellent or good in 92% and 89% following the new and standard regimens, respectively.
Conclusions: Single PDT with possible re-treatment of primary, superficial, and nodular ≤ 2-mm-thick BCC was significantly less effective than the approved standard double treatment. The cosmetic outcome was favorable and comparable between the two treatment groups.
Copyright: © 2024 Christensen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Arits AH, Spoorenberg E, Mosterd K, Nelemans P, Kelleners-Smeets NW, Essers BA. Cost-effectiveness of topical imiquimod and fluorouracil vs. photodynamic therapy for treatment of superficial basal-cell carcinoma. Br J Dermatol. 2014;171(6):1501–7. Epub 20141028. doi: 10.1111/bjd.13066 . - DOI - PubMed
-
- Morton CA, Szeimies RM, Basset-Seguin N, Calzavara-Pinton P, Gilaberte Y, Haedersdal M, et al.. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: treatment delivery and established indications—actinic keratoses, Bowen’s disease and basal cell carcinomas. J Eur Acad Dermatol Venereol. 2019;33(12):2225–38. Epub 2019/11/30. doi: 10.1111/jdv.16017 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

