Quantum imaging of biological organisms through spatial and polarization entanglement
- PMID: 38457506
- PMCID: PMC10923495
- DOI: 10.1126/sciadv.adk1495
Quantum imaging of biological organisms through spatial and polarization entanglement
Abstract
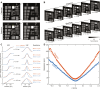
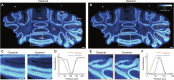
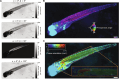
Quantum imaging holds potential benefits over classical imaging but has faced challenges such as poor signal-to-noise ratios, low resolvable pixel counts, difficulty in imaging biological organisms, and inability to quantify full birefringence properties. Here, we introduce quantum imaging by coincidence from entanglement (ICE), using spatially and polarization-entangled photon pairs to overcome these challenges. With spatial entanglement, ICE offers higher signal-to-noise ratios, greater resolvable pixel counts, and the ability to image biological organisms. With polarization entanglement, ICE provides quantitative quantum birefringence imaging capability, where both the phase retardation and the principal refractive index axis angle of an object can be remotely and instantly quantified without changing the polarization states of the photons incident on the object. Furthermore, ICE enables 25 times greater suppression of stray light than classical imaging. ICE has the potential to pave the way for quantum imaging in diverse fields, such as life sciences and remote sensing.
Figures



References
-
- Schermelleh L., Ferrand A., Huser T., Eggeling C., Sauer M., Biehlmaier O., Drummen G. P. C., Super-resolution microscopy demystified. Nat. Cell Biol. 21, 72–84 (2019). - PubMed
-
- Liu T.-L., Upadhyayula S., Milkie D. E., Singh V., Wang K., Swinburne I. A., Mosaliganti K. R., Collins Z. M., Hiscock T. W., Shea J., Kohrman A. Q., Medwig T. N., Dambournet D., Forster R., Cunniff B., Ruan Y., Yashiro H., Scholpp S., Meyerowitz E. M., Hockemeyer D., Drubin D. G., Martin B. L., Matus D. Q., Koyama M., Megason S. G., Kirchhausen T., Betzig E., Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science 360, eaaq1392 (2018). - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

