Identification of early risk factors for anti-citrullinated-protein-antibody positive rheumatoid arthritis-a prospective cohort study
- PMID: 38457608
- PMCID: PMC11534094
- DOI: 10.1093/rheumatology/keae146
Identification of early risk factors for anti-citrullinated-protein-antibody positive rheumatoid arthritis-a prospective cohort study
Abstract
Objective: Individuals positive for anti-cyclic-peptide-antibodies (anti-CCP) and musculoskeletal complaints (MSK-C) are at risk for developing rheumatoid arthritis (RA). In this study we aimed to investigate factors involved in arthritis progression.
Methods: Anti-CCP2-positive individuals with MSK-C referred to a rheumatologist were recruited. Individuals lacked arthritis at clinical and ultrasound examination and were followed for ≥3 years or until clinical arthritis diagnosis. Blood samples from inclusion were analysed for nine ACPA reactivities (citrullinated α-1-enolase, fibrinogen, filaggrin, histone, vimentin and tenascin peptides); 92 inflammation-associated proteins; and HLA-shared epitope alleles. Cox regression was applied to the data to identify independent predictors in a model.
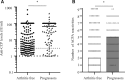
Results: Two hundred and sixty-seven individuals were included with median follow-up of 49 months (interquartile range [IQR]: 22-60); 101 (38%) developed arthritis after a median of 14 months (IQR: 6-27). The analysis identified that presence of at least one ACPA reactivity (hazard ratio [HR] 8.0; 95% CI: 2.9, 22), ultrasound-detected tenosynovitis (HR 3.4; 95% CI: 2.0, 6.0), IL-6 levels (HR 1.5; 95% CI: 1.2, 1.8) and IL-15 receptor α (IL-15Rα) levels (HR 0.6; 95% CI: 0.4, 0.9) are significant independent predictors for arthritis progression in a prediction model (Harrell's C 0.76 [s.e. 0.02], AUC 0.82 [95% CI: 0.76, 0.89], cross-validated AUC 0.70 [95% CI: 0.56, 0.85]).
Conclusion: We propose a high RA risk phase characterized by presence of ACPA reactivity, tenosynovitis, IL-6 and IL-15Rα and suggest that these factors need to be further investigated for their biological effects and clinical values, to identify individuals at particular low risk and high risk for arthritis progression.
Keywords: anti-citrullinated protein antibody reactivities; biomarkers; rheumatoid arthritis; risk phase.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures



References
-
- Hensvold AH, Magnusson PK, Joshua V et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: an epidemiological investigation in twins. Ann Rheum Dis 2015;74:375–80. - PubMed
-
- Jiang X, Frisell T, Askling J et al. To what extent is the familial risk of rheumatoid arthritis explained by established rheumatoid arthritis risk factors? Arthritis Rheumatol 2015;67:352–62. - PubMed
-
- Aletaha D, Neogi T, Silman AJ et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. - PubMed
-
- Brink M, Hansson M, Mathsson L et al. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to development of rheumatoid arthritis. Arthritis Rheum 2013;65:899–910. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

