Postpolymerization Modification of Poly(2-vinyl-4,4-dimethyl azlactone) as a Versatile Strategy for Drug Conjugation and Stimuli-Responsive Release
- PMID: 38457653
- PMCID: PMC11194783
- DOI: 10.1021/acs.biomac.4c00181
Postpolymerization Modification of Poly(2-vinyl-4,4-dimethyl azlactone) as a Versatile Strategy for Drug Conjugation and Stimuli-Responsive Release
Abstract
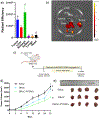
Postpolymerization modification of highly defined "scaffold" polymers is a promising approach for overcoming the existing limitations of controlled radical polymerization such as batch-to-batch inconsistencies, accessibility to different monomers, and compatibility with harsh synthesis conditions. Using multiple physicochemical characterization techniques, we demonstrate that poly(2-vinyl-4,4-dimethyl azlactone) (PVDMA) scaffolds can be efficiently modified with a coumarin derivative, doxorubicin, and camptothecin small molecule drugs. Subsequently, we show that coumarin-modified PVDMA has a high cellular biocompatibility and that coumarin derivatives are liberated from the polymer in the intracellular environment for cytosolic accumulation. In addition, we report the pharmacokinetics, biodistribution, and antitumor efficacy of a PVDMA-based polymer for the first time, demonstrating unique accumulation patterns based on the administration route (i.e., intravenous vs oral), efficient tumor uptake, and tumor growth inhibition in 4T1 orthotopic triple negative breast cancer (TNBC) xenografts. This work establishes the utility of PVDMA as a versatile chemical platform for producing polymer-drug conjugates with a tunable, stimuli-responsive delivery.
Figures







References
-
- Pedone E, Li X, Koseva N, Alpar O, Brocchini S, An information rich biomedical polymer library, J Mater. Chem 13(11) (2003) 2825–2837, 10.1039/B306857A. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

