Human papillomavirus-16 E6 activates the pentose phosphate pathway to promote cervical cancer cell proliferation by inhibiting G6PD lactylation
- PMID: 38457903
- PMCID: PMC10937312
- DOI: 10.1016/j.redox.2024.103108
Human papillomavirus-16 E6 activates the pentose phosphate pathway to promote cervical cancer cell proliferation by inhibiting G6PD lactylation
Abstract
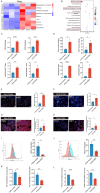
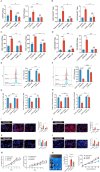
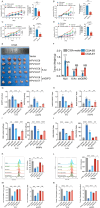
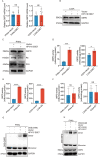
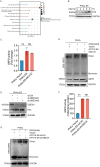
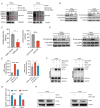
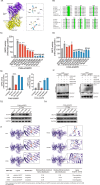
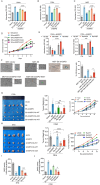
High-risk human papillomaviruses (HPVs) are the causative agents of cervical cancer. Here, we report that HPV16 E6E7 promotes cervical cancer cell proliferation by activating the pentose phosphate pathway (PPP). We found that HPV16 E6 activates the PPP primarily by increasing glucose-6-phosphate dehydrogenase (G6PD) enzyme activity. Mechanistically, HPV16 E6 promoted G6PD dimer formation by inhibiting its lactylation. Importantly, we suggest that G6PD K45 was lactylated during G6PD-mediated antioxidant stress. In primary human keratinocytes and an HPV-negative cervical cancer C33A cells line ectopically expressing HPV16 E6, the transduction of G6PD K45A (unable to be lactylated) increased GSH and NADPH levels and, correspondingly, decreasing ROS levels. Conversely, the re-expression of G6PD K45T (mimicking constitutive lactylation) in HPV16-positive SiHa cells line inhibited cell proliferation. In vivo, the inhibition of G6PD enzyme activity with 6-aminonicotinamide (6-An) or the re-expression of G6PD K45T inhibited tumor proliferation. In conclusion, we have revealed a novel mechanism of HPV oncoprotein-mediated malignant transformation. These findings might provide effective strategies for treating cervical and HPV-associated cancers.
Keywords: High-risk human papillomaviruses; Lactylation; Pentose phosphate pathway; glucose-6-phosphate dehydrogenase.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Human papillomavirus type 16 E6 promotes cervical cancer proliferation by upregulating transketolase enzymatic activity through the activation of protein kinase B.Mol Carcinog. 2024 Feb;63(2):339-355. doi: 10.1002/mc.23656. Epub 2023 Nov 21. Mol Carcinog. 2024. PMID: 37988232
-
Dysregulation of G6PD by HPV E6 exacerbates cervical cancer by activating the STAT3/PLOD2 pathway.Carcinogenesis. 2025 Apr 3;46(2):bgaf005. doi: 10.1093/carcin/bgaf005. Carcinogenesis. 2025. PMID: 39943705
-
Human Papillomavirus 16 Oncoprotein Expression Is Controlled by the Cellular Splicing Factor SRSF2 (SC35).J Virol. 2015 May;89(10):5276-87. doi: 10.1128/JVI.03434-14. Epub 2015 Feb 25. J Virol. 2015. PMID: 25717103 Free PMC article.
-
The Emerging Roles of the Metabolic Regulator G6PD in Human Cancers.Int J Mol Sci. 2023 Dec 7;24(24):17238. doi: 10.3390/ijms242417238. Int J Mol Sci. 2023. PMID: 38139067 Free PMC article. Review.
-
Glucose-6-phosphate dehydrogenase, NADPH, and cell survival.IUBMB Life. 2012 May;64(5):362-9. doi: 10.1002/iub.1017. Epub 2012 Mar 20. IUBMB Life. 2012. PMID: 22431005 Free PMC article. Review.
Cited by
-
The emerging role of protein L-lactylation in metabolic regulation and cell signalling.Nat Metab. 2025 Apr;7(4):647-664. doi: 10.1038/s42255-025-01259-0. Epub 2025 Apr 2. Nat Metab. 2025. PMID: 40175761 Review.
-
Lactylation and viral infections: A novel link between metabolic reprogramming and immune regulation.PLoS Pathog. 2025 Jul 28;21(7):e1013366. doi: 10.1371/journal.ppat.1013366. eCollection 2025 Jul. PLoS Pathog. 2025. PMID: 40720394 Free PMC article. Review.
-
Feline Calicivirus Infection Manipulates Central Carbon Metabolism.Vet Sci. 2025 Feb 7;12(2):138. doi: 10.3390/vetsci12020138. Vet Sci. 2025. PMID: 40005898 Free PMC article.
-
Lactate metabolism and lactylation in breast cancer: mechanisms and implications.Cancer Metastasis Rev. 2025 Apr 28;44(2):48. doi: 10.1007/s10555-025-10264-4. Cancer Metastasis Rev. 2025. PMID: 40295451 Free PMC article. Review.
-
Lactylation: From Homeostasis to Pathological Implications and Therapeutic Strategies.MedComm (2020). 2025 May 29;6(6):e70226. doi: 10.1002/mco2.70226. eCollection 2025 Jun. MedComm (2020). 2025. PMID: 40443721 Free PMC article. Review.
References
-
- Schiffman M., et al. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016;2 - PubMed
-
- Tommasino M. The human papillomavirus family and its role in carcinogenesis. Semin. Cancer Biol. 2014;26:13–21. - PubMed
-
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

