Reasons for multiple biologic and targeted synthetic DMARD switching and characteristics of treatment refractory rheumatoid arthritis
- PMID: 38457949
- PMCID: PMC11088978
- DOI: 10.1016/j.semarthrit.2024.152421
Reasons for multiple biologic and targeted synthetic DMARD switching and characteristics of treatment refractory rheumatoid arthritis
Abstract
Objective: Switching biologic and targeted synthetic DMARD (b/tsDMARD) medications occurs commonly in RA patients, however data are limited on the reasons for these changes. The objective of the study was to identify and categorize reasons for b/tsDMARD switching and investigate characteristics associated with treatment refractory RA.
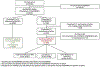
Methods: In a multi-hospital RA electronic health record (EHR) cohort, we identified RA patients prescribed ≥1 b/tsDMARD between 2001 and 2017. Consistent with the EULAR "difficult to treat" (D2T) RA definition, we further identified patients who discontinued ≥2 b/tsDMARDs with different mechanisms of action. We performed manual chart review to determine reasons for medication discontinuation. We defined "treatment refractory" RA as not achieving low disease activity (<3 tender or swollen joints on <7.5 mg of daily prednisone equivalent) despite treatment with two different b/tsDMARD mechanisms of action. We compared demographic, lifestyle, and clinical factors between treatment refractory RA and b/tsDMARD initiators not meeting D2T criteria.
Results: We identified 6040 RA patients prescribed ≥1 b/tsDMARD including 404 meeting D2T criteria. The most common reasons for medication discontinuation were inadequate response (43.3 %), loss of efficacy (25.8 %), and non-allergic adverse events (13.7 %). Of patients with D2T RA, 15 % had treatment refractory RA. Treatment refractory RA patients were younger at b/tsDMARD initiation (mean 47.2 vs. 55.2 years, p < 0.001), more commonly female (91.8% vs. 76.1 %, p = 0.006), and ever smokers (68.9% vs. 49.9 %, p = 0.005). No RA clinical factors differentiated treatment refractory RA patients from b/tsDMARD initiators.
Conclusions: In a large EHR-based RA cohort, the most common reasons for b/tsDMARD switching were inadequate response, loss of efficacy, and nonallergic adverse events (e.g. infections, leukopenia, psoriasis). Clinical RA factors were insufficient for differentiating b/tsDMARD responders from nonresponders.
Keywords: Biological therapy; Disease modifying antirheumatic drugs; Rheumatoid arthritis.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest MEW reports research support from Bristol Myers Squibb, Aqtual, Abbvie, and Janssen; consultancy for Abbvie, Aclaris, Amgen, Aqtual, Bristol Myers Squibb, CorEvitas, Glaxo Smith Kline, Gilead, Horizon, Johnson & Johnson, Lilly, Pfizer, Rani, Revolo, Saniofi, Scipher, Sci Rhom, Set Point, and Tremeau; and stock or stock options from Canfite, Inmedix, Scipher. Other authors report no competing interests.
Figures
References
-
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. The Lancet 2016;388:2023–2038. - PubMed
-
- Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet 2017;389:2338–2348. - PubMed
-
- Buch MH, Eyre S, McGonagle D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat Rev Rheumatol 2021;17:17–33. - PubMed
-
- Roodenrijs NMT, Hair MJH de, Goes MC van der, et al. Characteristics of difficult-to-treat rheumatoid arthritis: results of an international survey. Ann Rheum Dis 2018;77:1705–1709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical