Recent advances in ratiometric fluorescence imaging of enzyme activity in vivo
- PMID: 38457961
- PMCID: PMC11164639
- DOI: 10.1016/j.cbpa.2024.102441
Recent advances in ratiometric fluorescence imaging of enzyme activity in vivo
Abstract
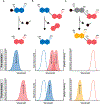
Among molecular imaging modalities that can monitor enzyme activity in vivo, optical imaging provides sensitive, molecular-level information at low-cost using safe and non-ionizing wavelengths of light. Yet, obtaining quantifiable optical signals in vivo poses significant challenges. Benchmarking using ratiometric signals can overcome dependence on dosing, illumination variability, and pharmacokinetics to provide quantitative in vivo optical data. This review highlights recent advances using fluorescent probes that are processed by enzymes to induce photophysical changes that can be monitored by ratiometric imaging. These diverse strategies include caged fluorophores that change photophysical properties upon enzymatic cleavage, as well as multi-fluorophore systems that are triggered by enzymatic cleavage to alter optical outputs in one or more fluorescent channels. The strategies discussed here have great potential for further development as well as potential broad applications for targeting diverse enzymes important for a wide range of human diseases.
Keywords: Enzyme probes; Fluorescent probes; Optical imaging.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

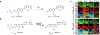


Similar articles
-
Enzyme-Activated Fluorogenic Probes for Live-Cell and in Vivo Imaging.ACS Chem Biol. 2018 Jul 20;13(7):1810-1823. doi: 10.1021/acschembio.8b00371. Epub 2018 Jul 6. ACS Chem Biol. 2018. PMID: 29924581 Free PMC article. Review.
-
Ratiometric Optical and Photoacoustic Imaging In Vivo in the Second Near-Infrared Window.Acc Chem Res. 2023 Nov 21;56(22):3223-3234. doi: 10.1021/acs.accounts.3c00495. Epub 2023 Nov 7. Acc Chem Res. 2023. PMID: 37935043
-
Recent advances in minimal fluorescent probes for optical imaging.Curr Opin Chem Biol. 2024 Jun;80:102458. doi: 10.1016/j.cbpa.2024.102458. Epub 2024 Apr 25. Curr Opin Chem Biol. 2024. PMID: 38670028 Review.
-
Molecular probes for fluorescence lifetime imaging.Bioconjug Chem. 2015 Jun 17;26(6):963-74. doi: 10.1021/acs.bioconjchem.5b00167. Epub 2015 May 22. Bioconjug Chem. 2015. PMID: 25961514 Free PMC article. Review.
-
Recent Advances in the Development of Optical Imaging Probes for γ-Glutamyltranspeptidase.Chembiochem. 2019 Feb 15;20(4):474-487. doi: 10.1002/cbic.201800370. Epub 2018 Sep 19. Chembiochem. 2019. PMID: 30062708 Review.
Cited by
-
Progress in the Application of Fluorescent Probes for Surgical Navigation in Breast Cancer Models.ACS Omega. 2025 Jul 25;10(30):32637-32650. doi: 10.1021/acsomega.5c04607. eCollection 2025 Aug 5. ACS Omega. 2025. PMID: 40787401 Free PMC article. Review.
References
-
- Zmudzinski M, Malon O, Poręba M, Drąg M, Imaging of proteases using activity-based probes, Curr. Opin. Chem. Biol 74 (2023) 102299. - PubMed
-
- James ML, Gambhir SS, A molecular imaging primer: modalities, imaging agents, and applications. Physiol. Rev 92 (2012), 897–965. - PubMed
-
- Louie AY, Hüber MM, Ahrens ET, Rothbächer U, Moats R, Jacobs RE, Fraser SE, Meade TJ, In vivo visualization of gene expression using magnetic resonance imaging, Nat. Biotechnol 18 (2000) 321–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

