Mast cell degranulation-triggered by SARS-CoV-2 induces tracheal-bronchial epithelial inflammation and injury
- PMID: 38458399
- PMCID: PMC11074640
- DOI: 10.1016/j.virs.2024.03.001
Mast cell degranulation-triggered by SARS-CoV-2 induces tracheal-bronchial epithelial inflammation and injury
Abstract
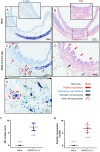
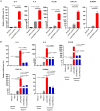
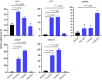
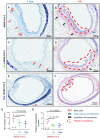
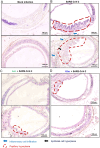
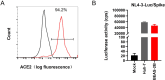
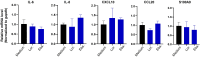
SARS-CoV-2 infection-induced hyper-inflammation is a key pathogenic factor of COVID-19. Our research, along with others', has demonstrated that mast cells (MCs) play a vital role in the initiation of hyper-inflammation caused by SARS-CoV-2. In previous study, we observed that SARS-CoV-2 infection induced the accumulation of MCs in the peri-bronchus and bronchioalveolar-duct junction in humanized mice. Additionally, we found that MC degranulation triggered by the spike protein resulted in inflammation in alveolar epithelial cells and capillary endothelial cells, leading to subsequent lung injury. The trachea and bronchus are the routes for SARS-CoV-2 transmission after virus inhalation, and inflammation in these regions could promote viral spread. MCs are widely distributed throughout the respiratory tract. Thus, in this study, we investigated the role of MCs and their degranulation in the development of inflammation in tracheal-bronchial epithelium. Histological analyses showed the accumulation and degranulation of MCs in the peri-trachea of humanized mice infected with SARS-CoV-2. MC degranulation caused lesions in trachea, and the formation of papillary hyperplasia was observed. Through transcriptome analysis in bronchial epithelial cells, we found that MC degranulation significantly altered multiple cellular signaling, particularly, leading to upregulated immune responses and inflammation. The administration of ebastine or loratadine effectively suppressed the induction of inflammatory factors in bronchial epithelial cells and alleviated tracheal injury in mice. Taken together, our findings confirm the essential role of MC degranulation in SARS-CoV-2-induced hyper-inflammation and the subsequent tissue lesions. Furthermore, our results support the use of ebastine or loratadine to inhibit SARS-CoV-2-triggered degranulation, thereby preventing tissue damage caused by hyper-inflammation.
Keywords: Bronchial epithelial cell; Inflammation; Mast cell (MC); SARS-CoV-2; Tracheal injury.
Copyright © 2024 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest. Prof. Ling Chen and Prof. Jian-Hua Wang are editorial board members for Virologica Sinica and were not involved in the editorial review or the decision to publish this article.
Figures

References
-
- Altmann D.M., Whettlock E.M., Liu S., Arachchillage D.J., Boyton R.J. The immunology of long COVID. Nat. Rev. Immunol. 2023;23:618–634. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

