Pathogenic variants affecting the TB5 domain of the fibrillin-1 protein: not only in geleophysic/acromicric dysplasias but also in Marfan syndrome
- PMID: 38458756
- PMCID: PMC11041597
- DOI: 10.1136/jmg-2023-109646
Pathogenic variants affecting the TB5 domain of the fibrillin-1 protein: not only in geleophysic/acromicric dysplasias but also in Marfan syndrome
Abstract
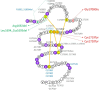
Background: Marfan syndrome (MFS) is a multisystem disease with a unique combination of skeletal, cardiovascular and ocular features. Geleophysic/acromicric dysplasias (GPHYSD/ACMICD), characterised by short stature and extremities, are described as 'the mirror image' of MFS. The numerous FBN1 pathogenic variants identified in MFS are located all along the gene and lead to the same final pathogenic sequence. Conversely, in GPHYSD/ACMICD, the 28 known heterozygous FBN1 pathogenic variants all affect exons 41-42 encoding TGFβ-binding protein-like domain 5 (TB5).
Methods: Since 1996, more than 5000 consecutive probands have been referred nationwide to our laboratory for molecular diagnosis of suspected MFS.
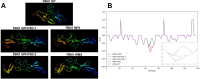
Results: We identified five MFS probands carrying distinct heterozygous pathogenic in-frame variants affecting the TB5 domain of FBN1. The clinical data showed that the probands displayed a classical form of MFS. Strikingly, one missense variant affects an amino acid that was previously involved in GPHYSD.
Conclusion: Surprisingly, pathogenic variants in the TB5 domain of FBN1 can lead to two opposite phenotypes: GPHYSD/ACMICD and MFS, suggesting the existence of different pathogenic sequences with the involvement of tissue specificity. Further functional studies are ongoing to determine the precise role of this domain in the physiopathology of each disease.
Keywords: Cardiovascular Diseases; Diagnosis; Genetic Diseases, Inborn; Musculoskeletal Diseases.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
