Comparing population-level humoral and cellular immunity to SARS-Cov-2 in Bangalore, India
- PMID: 38459035
- PMCID: PMC10923858
- DOI: 10.1038/s41598-024-54922-z
Comparing population-level humoral and cellular immunity to SARS-Cov-2 in Bangalore, India
Abstract
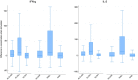
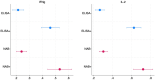
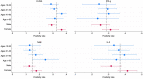
Two types of immunity, humoral and cellular, offer protection against COVID. Humoral protection, contributed by circulating neutralizing antibodies, can provide immediate protection but decays more quickly than cellular immunity and can lose effectiveness in the face of mutation and drift in the SARS-CoV-2 spike protein. Therefore, population-level seroprevalence surveys used to estimate population-level immunity may underestimate the degree to which a population is protected against COVID. In early 2021, before India began its vaccination campaign, we tested for humoral and cellular immunity to SARS-Cov-2 in representative samples of slum and non-slum populations in Bangalore, India. We found that 29.7% of samples (unweighted) had IgG antibodies to the spike protein and 15.5% had neutralizing antibodies, but at up to 46% showed evidence of cellular immunity. We also find that prevalence of cellular immunity is significantly higher in slums than in non-slums. These findings suggest (1) that a significantly larger proportion of the population in Bangalore, India, had cellular immunity to SARS-CoV-2 than had humoral immunity, as measured by serological surveys, and (2) that low socio-economic status communities display higher frequency of cellular immunity, likely because of greater exposure to infection due to population density.
© 2024. The Author(s).
Conflict of interest statement
AM reports consulting for the World Bank, the Asian Development Bank, and IDFC Institute (under a Rockefeller Foundation grant) during this study and conducting seroprevalence studies or training in locations other than Bangalore during the time of this study. The remaining authors report no potential or actual conflicts of interest.
Figures
Similar articles
-
Function Is More Reliable than Quantity to Follow Up the Humoral Response to the Receptor-Binding Domain of SARS-CoV-2-Spike Protein after Natural Infection or COVID-19 Vaccination.Viruses. 2021 Sep 30;13(10):1972. doi: 10.3390/v13101972. Viruses. 2021. PMID: 34696403 Free PMC article.
-
Humoral and T cell responses to SARS-CoV-2 reveal insights into immunity during the early pandemic period in Pakistan.BMC Infect Dis. 2023 Dec 1;23(1):846. doi: 10.1186/s12879-023-08829-1. BMC Infect Dis. 2023. PMID: 38041026 Free PMC article.
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
Characterization of SARS-CoV-2-specific humoral immunity and its potential applications and therapeutic prospects.Cell Mol Immunol. 2022 Feb;19(2):150-157. doi: 10.1038/s41423-021-00774-w. Epub 2021 Oct 13. Cell Mol Immunol. 2022. PMID: 34645940 Free PMC article. Review.
-
T follicular helper cells in the humoral immune response to SARS-CoV-2 infection and vaccination.J Leukoc Biol. 2022 Feb;111(2):355-365. doi: 10.1002/JLB.5MR0821-464R. Epub 2021 Nov 3. J Leukoc Biol. 2022. PMID: 34730247 Free PMC article. Review.
Cited by
-
SARS-CoV-2-specific humoral and cellular immunity assessment in Peruvian vaccinated population: a cross-sectional study.PeerJ. 2025 Jul 15;13:e19651. doi: 10.7717/peerj.19651. eCollection 2025. PeerJ. 2025. PMID: 40687756 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

