Clinical electromagnetic brain scanner
- PMID: 38459073
- PMCID: PMC10923816
- DOI: 10.1038/s41598-024-55360-7
Clinical electromagnetic brain scanner
Abstract
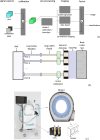
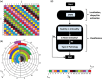
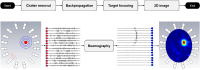
Stroke is a leading cause of death and disability worldwide, and early diagnosis and prompt medical intervention are thus crucial. Frequent monitoring of stroke patients is also essential to assess treatment efficacy and detect complications earlier. While computed tomography (CT) and magnetic resonance imaging (MRI) are commonly used for stroke diagnosis, they cannot be easily used onsite, nor for frequent monitoring purposes. To meet those requirements, an electromagnetic imaging (EMI) device, which is portable, non-invasive, and non-ionizing, has been developed. It uses a headset with an antenna array that irradiates the head with a safe low-frequency EM field and captures scattered fields to map the brain using a complementary set of physics-based and data-driven algorithms, enabling quasi-real-time detection, two-dimensional localization, and classification of strokes. This study reports clinical findings from the first time the device was used on stroke patients. The clinical results on 50 patients indicate achieving an overall accuracy of 98% in classification and 80% in two-dimensional quadrant localization. With its lightweight design and potential for use by a single para-medical staff at the point of care, the device can be used in intensive care units, emergency departments, and by paramedics for onsite diagnosis.
© 2024. Crown.
Conflict of interest statement
The co-authors, K.B. and S.C. have a partial appointment with the company, EMvision Medical Devices, which owns the IP of the project. All other authors declare that they do not have any competing interests.
Figures









References
-
- Mohammed B, Abbosh A, Mustafa S, Ireland D. Microwave system for head imaging. IEEE Trans. Instrum. Meas. 2014;63(1):117–123. doi: 10.1109/TIM.2013.2277562. - DOI
-
- Pastorino M, Randazzo A. Microwave Imaging Methods and Applications. Artech House; 2018.
MeSH terms
Grants and funding
- CRC-P60941/Australian Department of Industry, Innovation and Science, Cooperative Research Centres Projects (CRC-P) Grants
- CRC-P60941/Australian Department of Industry, Innovation and Science, Cooperative Research Centres Projects (CRC-P) Grants
- CRC-P60941/Australian Department of Industry, Innovation and Science, Cooperative Research Centres Projects (CRC-P) Grants
- CRC-P60941/Australian Department of Industry, Innovation and Science, Cooperative Research Centres Projects (CRC-P) Grants
- CRC-P60941/Australian Department of Industry, Innovation and Science, Cooperative Research Centres Projects (CRC-P) Grants
- CRC-P60941/Australian Department of Industry, Innovation and Science, Cooperative Research Centres Projects (CRC-P) Grants
- CRC-P60941/Australian Department of Industry, Innovation and Science, Cooperative Research Centres Projects (CRC-P) Grants
- CRC-P60941/Australian Department of Industry, Innovation and Science, Cooperative Research Centres Projects (CRC-P) Grants
- CRC-P60941/Australian Department of Industry, Innovation and Science, Cooperative Research Centres Projects (CRC-P) Grants
- CRC-P60941/Australian Department of Industry, Innovation and Science, Cooperative Research Centres Projects (CRC-P) Grants
LinkOut - more resources
Full Text Sources
Medical

