Microfluidic Diffusional Sizing (MDS) Measurements of Secretory Neutralizing Antibody Affinity Against SARS-CoV-2
- PMID: 38459195
- PMCID: PMC11082020
- DOI: 10.1007/s10439-024-03478-0
Microfluidic Diffusional Sizing (MDS) Measurements of Secretory Neutralizing Antibody Affinity Against SARS-CoV-2
Abstract
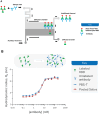
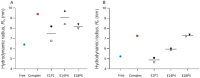
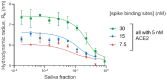
SARS-CoV-2 has rampantly spread around the globe and continues to cause unprecedented loss through ongoing waves of (re)infection. Increasing our understanding of the protection against infection with SARS-CoV-2 is critical to ending the pandemic. Serological assays have been widely used to assess immune responses, but secretory antibodies, the essential first line of defense, have been studied to only a limited extent. Of particular interest and importance are neutralizing antibodies, which block the binding of the spike protein of SARS-CoV-2 to the human receptor angiotensin-converting enzyme-2 (ACE2) and thus are essential for immune defense. Here, we employed Microfluidic Diffusional Sizing (MDS), an immobilization-free technology, to characterize neutralizing antibody affinity to SARS-CoV-2 spike receptor-binding domain (RBD) and spike trimer in saliva. Affinity measurement was obtained through a contrived sample and buffer using recombinant SARS-CoV-2 RBD and monoclonal antibody. Limited saliva samples demonstrated that MDS applies to saliva neutralizing antibody measurement. The ability to disrupt a complex of ACE2-Fc and spike trimer is shown. Using a quantitative assay on the patient sample, we determined the affinity and binding site concentration of the neutralizing antibodies.
Keywords: Microfluidic diffusional sizing; Neutralizing antibody; SARS-CoV-2; Saliva.
© 2024. The Author(s).
Figures


Similar articles
-
Structural Basis of a Human Neutralizing Antibody Specific to the SARS-CoV-2 Spike Protein Receptor-Binding Domain.Microbiol Spectr. 2021 Oct 31;9(2):e0135221. doi: 10.1128/Spectrum.01352-21. Epub 2021 Oct 13. Microbiol Spectr. 2021. PMID: 34643438 Free PMC article.
-
Competitive SARS-CoV-2 Serology Reveals Most Antibodies Targeting the Spike Receptor-Binding Domain Compete for ACE2 Binding.mSphere. 2020 Sep 16;5(5):e00802-20. doi: 10.1128/mSphere.00802-20. mSphere. 2020. PMID: 32938700 Free PMC article.
-
SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies.Nature. 2020 Dec;588(7839):682-687. doi: 10.1038/s41586-020-2852-1. Epub 2020 Oct 12. Nature. 2020. PMID: 33045718 Free PMC article.
-
Mutations in the SARS-CoV-2 spike receptor binding domain and their delicate balance between ACE2 affinity and antibody evasion.Protein Cell. 2024 May 28;15(6):403-418. doi: 10.1093/procel/pwae007. Protein Cell. 2024. PMID: 38442025 Free PMC article. Review.
-
Recognition of the SARS-CoV-2 receptor binding domain by neutralizing antibodies.Biochem Biophys Res Commun. 2021 Jan 29;538:192-203. doi: 10.1016/j.bbrc.2020.10.012. Epub 2020 Oct 10. Biochem Biophys Res Commun. 2021. PMID: 33069360 Free PMC article. Review.
References
-
- Ania W, Fatima A, Adolfo F, Mayce M, Meagan M, Philip M, Rao MD, Kimberly M, Daniel S, Kimberly S, Shirin S, Viviana S, Judith A, Florian K, Carlos CC. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. - DOI - PMC - PubMed
-
- Arosio P, Müller T, Rajah L, Yates EV, Aprile FA, Zhang Y, Cohen SIA, White DA, Herling TW, De Genst EJ, Linse S, Vendruscolo M, Dobson CM, Knowles TPJ. Microfluidic diffusion analysis of the sizes and interactions of proteins under native solution conditions. ACS Nano. 2016;10:333–341. doi: 10.1021/acsnano.5b04713. - DOI - PubMed
-
- Beeg M, Nobili A, Orsini B, Rogai F, Gilardi D, Fiorino G, Danese S, Salmona M, Garattini S, Gobbi M. A Surface Plasmon Resonance-based assay to measure serum concentrations of therapeutic antibodies and anti-drug antibodies. Sci. Rep. 2019;9(1):1–9. doi: 10.1038/s41598-018-37950-4. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

