Role of Glucocorticoids in Metabolic Dysfunction-Associated Steatotic Liver Disease
- PMID: 38459229
- PMCID: PMC11150302
- DOI: 10.1007/s13679-024-00556-1
Role of Glucocorticoids in Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
Purpose of the review: To summarize published data on the association between glucocorticoids and metabolic dysfunction-associated steatotic liver disease (MASLD), focusing on the possible pathophysiological links and related treatment considerations.
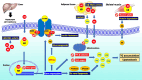
Recent findings: Glucocorticoids, commonly used for managing many inflammatory and autoimmune diseases, may contribute to the development and progression of MASLD. Glucocorticoids may induce hyperglycemia and hyperinsulinemia, thus increasing systemic and hepatic insulin resistance, a hallmark of MASLD pathogenesis. Furthermore, glucocorticoids increase adipose tissue lipolysis, and hepatic de novo lipogenesis and decrease hepatic fatty acid β-oxidation, thus promoting MASLD development. Preclinical evidence also suggests that glucocorticoids may adversely affect hepatic inflammation and fibrosis. 11beta-hydroxysteroid dehydrogenase type 1 (11β-HSD1) and 5α-reductase are implicated in the link between glucocorticoids and MASLD, the former enzyme increasing and the latter reducing the glucocorticoid action on the liver. Treatment considerations exist due to the pathogenic link between glucocorticoids and MASLD. Since iatrogenic hypercortisolism is common, glucocorticoids should be used at the minimum daily dose to control the subjective disease. Furthermore, the pharmacologic inhibition of 11β-HSD1 has provided favorable results in MASLD, both in preclinical studies and early MASH clinical trials. Glucocorticoids are closely linked to MASLD pathophysiology, with specific clinical and therapeutic implications.
Keywords: Adrenals; Cortisol; Glucocorticoids; Metabolic dysfunction-associated steatohepatitis; Metabolic dysfunction-associated steatotic liver disease; Nonalcoholic fatty liver disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

