Porphyrin-fused graphene nanoribbons
- PMID: 38459234
- PMCID: PMC11230900
- DOI: 10.1038/s41557-024-01477-1
Porphyrin-fused graphene nanoribbons
Abstract
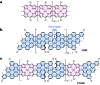
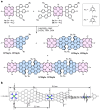
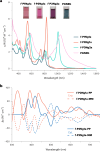
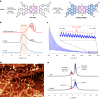
Graphene nanoribbons (GNRs), nanometre-wide strips of graphene, are promising materials for fabricating electronic devices. Many GNRs have been reported, yet no scalable strategies are known for synthesizing GNRs with metal atoms and heteroaromatic units at precisely defined positions in the conjugated backbone, which would be valuable for tuning their optical, electronic and magnetic properties. Here we report the solution-phase synthesis of a porphyrin-fused graphene nanoribbon (PGNR). This PGNR has metalloporphyrins fused into a twisted fjord-edged GNR backbone; it consists of long chains (>100 nm), with a narrow optical bandgap (~1.0 eV) and high local charge mobility (>400 cm2 V-1 s-1 by terahertz spectroscopy). We use this PGNR to fabricate ambipolar field-effect transistors with appealing switching behaviour, and single-electron transistors displaying multiple Coulomb diamonds. These results open an avenue to π-extended nanostructures with engineerable electrical and magnetic properties by transposing the coordination chemistry of porphyrins into graphene nanoribbons.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Nakada K, Fujita M, Dresselhaus G, Dresselhaus MS. Edge state in graphene ribbons: nanometer size effect and edge shape dependence. Phys. Rev. B. 1996;54:17954–17961. - PubMed
-
- Son YW, Cohen ML, Louie SG. Energy gaps in graphene nanoribbons. Phys. Rev. Lett. 2006;97:216803. - PubMed
-
- Wang H, et al. Graphene nanoribbons for quantum electronics. Nat. Rev. Phys. 2021;3:791–802.
-
- Jiao L, Wang X, Diankov G, Wang H, Dai H. Facile synthesis of high-quality graphene nanoribbons. Nat. Nanotechnol. 2010;5:321–325. - PubMed
Grants and funding
- 885606 ARO-MAT/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- ERC-CoG-773048-MMGNR/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- Pathfinder-101099676-4D-NMR/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- EP/N014995/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
- EP/N017188/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

