Dectin-1 aggravates neutrophil inflammation through caspase-11/4-mediated macrophage pyroptosis in asthma
- PMID: 38459541
- PMCID: PMC10921740
- DOI: 10.1186/s12931-024-02743-z
Dectin-1 aggravates neutrophil inflammation through caspase-11/4-mediated macrophage pyroptosis in asthma
Abstract
Background: The pattern recognition receptor Dectin-1 was initially discovered to play a pivotal role in mediating pulmonary antifungal immunity and promoting neutrophil-driven inflammation. Recent studies have revealed that Dectin-1 is overexpressed in asthma, but the specific mechanism remains elusive. Additionally, Dectin-1 has been implicated in promoting pyroptosis, a hallmark of severe asthma airway inflammation. Nevertheless, the involvement of the non-classical pyroptosis signal caspase-11/4 and its upstream regulatory mechanisms in asthma has not been completely explored.
Methods: House dust mite (HDM)-induced mice was treated with Dectin-1 agonist Curdlan, Dectin-1 inhibitor Laminarin, and caspase-11 inhibitor wedelolactone separately. Subsequently, inflammatory cells in bronchoalveolar lavage fluid (BALF) were analyzed. Western blotting was performed to measure the protein expression of caspase-11 and gasdermin D (GSDMD). Cell pyroptosis and the expression of chemokine were detected in vitro. The correlation between Dectin-1 expression, pyroptosis factors and neutrophils in the induced sputum of asthma patients was analyzed.
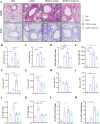
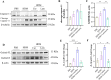
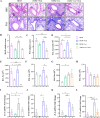
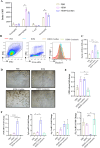
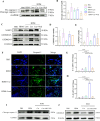
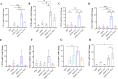
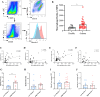
Results: Curdlan appeared to exacerbate neutrophil airway inflammation in asthmatic mice, whereas wedelolactone effectively alleviated airway inflammation aggravated by Curdlan. Moreover, Curdlan enhanced the release of caspase-11 activation fragments and N-terminal fragments of gasdermin D (GSDMD-N) stimulated by HDM both in vivo or in vitro. In mouse alveolar macrophages (MH-S cells), Curdlan/HDM stimulation resulted in vacuolar degeneration and elevated lactate dehydrogenase (LDH) release. In addition, there was an upregulation of neutrophil chemokines CXCL1, CXCL3, CXCL5 and their receptor CXCR2, which was suppressed by wedelolactone. In asthma patients, a positive correlation was observed between the expression of Dectin-1 on macrophages and caspase-4 (the human homology of caspase-11), and the proportion of neutrophils in induced sputum.
Conclusion: Dectin-1 activation in asthma induced caspase-11/4 mediated macrophage pyroptosis, which subsequently stimulated the secretion of chemokines, leading to the exacerbation of airway neutrophil inflammation.
Keywords: Asthma; Caspase-11; Dectin-1; Neutrophil; Pyroptosis.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

References
-
- Hinks TSC, Brown T, Lau LCK, Rupani H, Barber C, et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol. 2016;138(1):61–75. doi: 10.1016/j.jaci.2015.11.020. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 2022JJ30924/Natural Science Foundation of Hunan Province,China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 82270033/National Natural Science Foundation of China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
- 81873407/National Natural Science Foundation of China,China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

