TRF2 as novel marker of tumor response to taxane-based therapy: from mechanistic insight to clinical implication
- PMID: 38459559
- PMCID: PMC10924347
- DOI: 10.1186/s13046-024-02998-w
TRF2 as novel marker of tumor response to taxane-based therapy: from mechanistic insight to clinical implication
Abstract
Background: Breast Cancer (BC) can be classified, due to its heterogeneity, into multiple subtypes that differ for prognosis and clinical management. Notably, triple negative breast cancer (TNBC) - the most aggressive BC form - is refractory to endocrine and most of the target therapies. In this view, taxane-based therapy still represents the elective strategy for the treatment of this tumor. However, due variability in patients' response, management of TNBC still represents an unmet medical need. Telomeric Binding Factor 2 (TRF2), a key regulator of telomere integrity that is over-expressed in several tumors, including TNBC, has been recently found to plays a role in regulating autophagy, a degradative process that is involved in drug detoxification. Based on these considerations, we pointed, here, at investigating if TRF2, regulating autophagy, can affect tumor sensitivity to therapy.
Methods: Human TNBC cell lines, over-expressing or not TRF2, were subjected to treatment with different taxanes and drug efficacy was tested in terms of autophagic response and cell proliferation. Autophagy was evaluated first biochemically, by measuring the levels of LC3, and then by immunofluorescence analysis of LC3-puncta positive cells. Concerning the proliferation, cells were subjected to colony formation assays associated with western blot and FACS analyses. The obtained results were then confirmed also in mouse models. Finally, the clinical relevance of our findings was established by retrospective analysis on a cohort of TNBC patients subjected to taxane-based neoadjuvant chemotherapy.
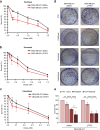
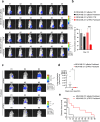
Results: This study demonstrated that TRF2, inhibiting autophagy, is able to increase the sensitivity of TNBC cells to taxanes. The data, first obtained in in vitro models, were then recapitulated in preclinical mouse models and in a cohort of TNBC patients, definitively demonstrating that TRF2 over-expression enhances the efficacy of taxane-based neoadjuvant therapy in reducing tumor growth and its recurrence upon surgical intervention.
Conclusions: Based on our finding it is possible to conclude that TRF2, already known for its role in promoting tumor formation and progression, might represents an Achilles' heel for cancer. In this view, TRF2 might be exploited as a putative biomarker to predict the response of TNBC patients to taxane-based neoadjuvant chemotherapy.
Keywords: Autophagy; Drug sensitivity; Predictive marker; TNBC; TRF2; Taxanes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

