A glutamine tug-of-war between cancer and immune cells: recent advances in unraveling the ongoing battle
- PMID: 38459595
- PMCID: PMC10921613
- DOI: 10.1186/s13046-024-02994-0
A glutamine tug-of-war between cancer and immune cells: recent advances in unraveling the ongoing battle
Erratum in
-
Correction: A glutamine tug-of-war between cancer and immune cells: recent advances in unraveling the ongoing battle.J Exp Clin Cancer Res. 2024 Mar 26;43(1):93. doi: 10.1186/s13046-024-03018-7. J Exp Clin Cancer Res. 2024. PMID: 38532446 Free PMC article. No abstract available.
Abstract
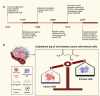
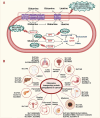
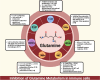
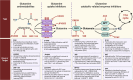
Glutamine metabolism plays a pivotal role in cancer progression, immune cell function, and the modulation of the tumor microenvironment. Dysregulated glutamine metabolism has been implicated in cancer development and immune responses, supported by mounting evidence. Cancer cells heavily rely on glutamine as a critical nutrient for survival and proliferation, while immune cells require glutamine for activation and proliferation during immune reactions. This metabolic competition creates a dynamic tug-of-war between cancer and immune cells. Targeting glutamine transporters and downstream enzymes involved in glutamine metabolism holds significant promise in enhancing anti-tumor immunity. A comprehensive understanding of the intricate molecular mechanisms underlying this interplay is crucial for developing innovative therapeutic approaches that improve anti-tumor immunity and patient outcomes. In this review, we provide a comprehensive overview of recent advances in unraveling the tug-of-war of glutamine metabolism between cancer and immune cells and explore potential applications of basic science discoveries in the clinical setting. Further investigations into the regulation of glutamine metabolism in cancer and immune cells are expected to yield valuable insights, paving the way for future therapeutic interventions.
Keywords: Cancer; Glutamine metabolism; Immune cells; Therapeutic strategies; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Exploiting pancreatic cancer metabolism: challenges and opportunities.Trends Mol Med. 2024 Jun;30(6):592-604. doi: 10.1016/j.molmed.2024.03.008. Epub 2024 Apr 10. Trends Mol Med. 2024. PMID: 38604929 Review.
-
Targeting glutamine metabolism crosstalk with tumor immune response.Biochim Biophys Acta Rev Cancer. 2025 Feb;1880(1):189257. doi: 10.1016/j.bbcan.2024.189257. Epub 2024 Dec 31. Biochim Biophys Acta Rev Cancer. 2025. PMID: 39746457 Review.
-
Glutamine Metabolism Regulators Associated with Cancer Development and the Tumor Microenvironment: A Pan-Cancer Multi-Omics Analysis.Genes (Basel). 2021 Aug 25;12(9):1305. doi: 10.3390/genes12091305. Genes (Basel). 2021. PMID: 34573287 Free PMC article.
-
Glutamine and cancer: metabolism, immune microenvironment, and therapeutic targets.Cell Commun Signal. 2025 Jan 24;23(1):45. doi: 10.1186/s12964-024-02018-6. Cell Commun Signal. 2025. PMID: 39856712 Free PMC article. Review.
-
Reprogramming of glutamine metabolism and its impact on immune response in the tumor microenvironment.Cell Commun Signal. 2022 Jul 27;20(1):114. doi: 10.1186/s12964-022-00909-0. Cell Commun Signal. 2022. PMID: 35897036 Free PMC article. Review.
Cited by
-
Amino acid deprivation in cancer cells with compensatory autophagy induction increases sensitivity to autophagy inhibitors.Mol Cell Oncol. 2024 Jul 14;11(1):2377404. doi: 10.1080/23723556.2024.2377404. eCollection 2024. Mol Cell Oncol. 2024. PMID: 39021618 Free PMC article.
-
Fuel for thought: targeting metabolism in lung cancer.Transl Lung Cancer Res. 2024 Dec 31;13(12):3692-3717. doi: 10.21037/tlcr-24-662. Epub 2024 Dec 24. Transl Lung Cancer Res. 2024. PMID: 39830762 Free PMC article. Review.
-
Correction: A glutamine tug-of-war between cancer and immune cells: recent advances in unraveling the ongoing battle.J Exp Clin Cancer Res. 2024 Mar 26;43(1):93. doi: 10.1186/s13046-024-03018-7. J Exp Clin Cancer Res. 2024. PMID: 38532446 Free PMC article. No abstract available.
-
Tumor Biology Hides Novel Therapeutic Approaches to Diffuse Large B-Cell Lymphoma: A Narrative Review.Int J Mol Sci. 2024 Oct 23;25(21):11384. doi: 10.3390/ijms252111384. Int J Mol Sci. 2024. PMID: 39518937 Free PMC article. Review.
-
Enhancing precision in cancer treatment: the role of gene therapy and immune modulation in oncology.Front Med (Lausanne). 2025 Jan 13;11:1527600. doi: 10.3389/fmed.2024.1527600. eCollection 2024. Front Med (Lausanne). 2025. PMID: 39871848 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 81627901/National Natural Science Foundation of China-China Academy of General Technology Joint Fund for Basic Research
- 81972863/National Natural Science Foundation of China-China Academy of General Technology Joint Fund for Basic Research
- 82030082/National Natural Science Foundation of China-China Academy of General Technology Joint Fund for Basic Research
LinkOut - more resources
Full Text Sources
Medical

