Chronic TNF exposure induces glucocorticoid-like immunosuppression in the alveolar macrophages of aged mice that enhances their susceptibility to pneumonia
- PMID: 38459711
- PMCID: PMC11296116
- DOI: 10.1111/acel.14133
Chronic TNF exposure induces glucocorticoid-like immunosuppression in the alveolar macrophages of aged mice that enhances their susceptibility to pneumonia
Abstract
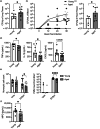
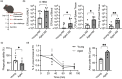
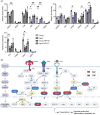
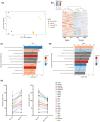
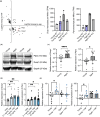
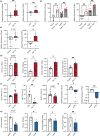
Chronic low-grade inflammation, particularly elevated tumor necrosis factor (TNF) levels, occurs due to advanced age and is associated with greater susceptibility to infection. One reason for this is age-dependent macrophage dysfunction (ADMD). Herein, we use the adoptive transfer of alveolar macrophages (AM) from aged mice into the airway of young mice to show that inherent age-related defects in AM were sufficient to increase the susceptibility to Streptococcus pneumoniae, a Gram-positive bacterium and the leading cause of community-acquired pneumonia. MAPK phosphorylation arrays using AM lysates from young and aged wild-type (WT) and TNF knockout (KO) mice revealed multilevel TNF-mediated suppression of kinase activity in aged mice. RNAseq analyses of AM validated the suppression of MAPK signaling as a consequence of TNF during aging. Two regulatory phosphatases that suppress MAPK signaling, Dusp1 and Ptprs, were confirmed to be upregulated with age and as a result of TNF exposure both ex vivo and in vitro. Dusp1 is known to be responsible for glucocorticoid-mediated immune suppression, and dexamethasone treatment increased Dusp1 and Ptprs expression in cells and recapitulated the ADMD phenotype. In young mice, treatment with dexamethasone increased the levels of Dusp1 and Ptprs and their susceptibility to infection. TNF-neutralizing antibody reduced Dusp1 and Ptprs levels in AM from aged mice and reduced pneumonia severity following bacterial challenge. We conclude that chronic exposure to TNF increases the expression of the glucocorticoid-associated MAPK signaling suppressors, Dusp1 and Ptprs, which inhibits AM activation and increases susceptibility to bacterial pneumonia in older adults.
Keywords: Streptococcus pneumoniae; Dusp1; MAPK; Ptprs; alveolar macrophages; glucocorticoids; inflamm‐aging; pneumonia; tumor necrosis factor.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
We have no conflicts of interest to declare.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Single nuclei RNA-sequencing unveils alveolar macrophages as drivers of endothelial damage in obese HFpEF-related pulmonary hypertension.Cardiovasc Diabetol. 2025 Jul 3;24(1):268. doi: 10.1186/s12933-025-02772-y. Cardiovasc Diabetol. 2025. PMID: 40611249 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Type I Interferon, Induced by Adenovirus or Adenoviral Vector Infection, Regulates the Cytokine Response to Lipopolysaccharide in a Macrophage Type-Specific Manner.J Innate Immun. 2024;16(1):226-247. doi: 10.1159/000538282. Epub 2024 Mar 25. J Innate Immun. 2024. PMID: 38527452 Free PMC article.
Cited by
-
Alterations in Nutrient Availability in the Lungs During Streptococcus pneumoniae-Induced Pneumonia.bioRxiv [Preprint]. 2025 Jul 14:2025.07.14.664699. doi: 10.1101/2025.07.14.664699. bioRxiv. 2025. PMID: 40791442 Free PMC article. Preprint.
References
-
- Abraham, S. M. , Lawrence, T. , Kleiman, A. , Warden, P. , Medghalchi, M. , Tuckermann, J. , Saklatvala, J. , & Clark, A. R. (2006). Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. Journal of Experimental Medicine, 203(8), 1883–1889. - PMC - PubMed
-
- Adrover, J. M. , Nicolás‐Ávila, J. A. , & Hidalgo, A. (2016). Aging: A temporal dimension for neutrophils. Trends in Immunology, 37(5), 334–345. - PubMed
-
- Bae, E. , Cha, R. H. , Kim, Y. C. , An, J. N. , Kim, D. K. , Yoo, K. D. , Lee, S. M. , Kim, M. H. , Park, J. T. , Kang, S. W. , Park, J. Y. , Lim, C. S. , Kim, Y. S. , Yang, S. H. , & Lee, J. P. (2017). Circulating TNF receptors predict cardiovascular disease in patients with chronic kidney disease. Medicine (Baltimore), 96(19), e6666. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

