Effect of Renal Impairment on Pharmacokinetics and Safety of Ensitrelvir, a SARS-CoV-2 3CL Protease Inhibitor
- PMID: 38460082
- PMCID: PMC10965878
- DOI: 10.1007/s40121-024-00946-x
Effect of Renal Impairment on Pharmacokinetics and Safety of Ensitrelvir, a SARS-CoV-2 3CL Protease Inhibitor
Erratum in
-
Correction to: Effect of Renal Impairment on Pharmacokinetics and Safety of Ensitrelvir, a SARS‑CoV‑2 3CL Protease Inhibitor.Infect Dis Ther. 2024 Sep;13(9):2103. doi: 10.1007/s40121-024-01030-0. Infect Dis Ther. 2024. PMID: 39136875 Free PMC article. No abstract available.
Abstract
Introduction: Ensitrelvir, a novel oral inhibitor of 3C-like protease of SARS-CoV-2, shows efficacy and safety in participants with mild to moderate COVID-19. Since urinary recovery of ensitrelvir ranged from 12.9% to 21.8% across dose groups given 20-1000 mg in a single-ascending dose study, renal excretion contributes to the elimination of ensitrelvir. Therefore, the effect of renal impairment on the pharmacokinetics and safety of ensitrelvir needed to be evaluated.
Methods: This study (NCT05363215) was a phase 1, open-label, nonrandomized, parallel-group study. The effect of renal function on the pharmacokinetics of ensitrelvir was investigated. Ensitrelvir was administered as a single dose of 375 mg to participants with normal renal function and those with mild, moderate, and severe renal impairment. The participants with normal renal function were matched to each participant with moderate renal impairment with respect to sex, age, and body mass index. The unbound fractions in plasma after administration of ensitrelvir were also evaluated. For the safety assessment, the nature, frequency, and severity of treatment-emergent adverse events were evaluated and recorded.
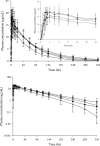
Results: The plasma concentrations of participants with renal impairment were higher than those of participants with normal renal function. The ratios (90% confidence intervals) of the area under the plasma concentration-time curve from 0 to infinity (AUC0-inf) in participants with mild, moderate, and severe renal impairment compared to normal renal function were 1.4374 (1.1716-1.7636), 1.4885 (1.1883-1.8646), and 1.6021 (1.2782-2.0080), respectively. The plasma protein-unbound fraction was similar regardless of the plasma ensitrelvir concentration or renal function. Ensitrelvir was well tolerated in participants with mild to severe renal impairment and normal renal function.
Conclusion: Ensitrelvir was well tolerated by participants with renal impairment. There was no clinically meaningful increase on exposure to ensitrelvir in participants with renal impairment, indicating that no dose adjustment would be required due to renal function.
Keywords: COVID-19; Ensitrelvir; Renal impairment study.
© 2024. The Author(s).
Conflict of interest statement
Takayuki Katsube, Ryosuke Shimizu, and Ryuji Kubota are employees of Shionogi & Co., Ltd. Kezbor Safwan is an employee of Shionogi Inc.
Figures
References
-
- Mukae H, Yotsuyanagi H, Ohmagari N, et al. Efficacy and safety of ensitrelvir in patients With mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study. Clin Infect Dis. 2023;76(8):1403–11. 10.1093/cid/ciac933. 10.1093/cid/ciac933 - DOI - PMC - PubMed
-
- Xocova® (Ensitrelvir Fumaric Acid) tablets 125 mg approved in Japan for the treatment of SARS-CoV-2 infection, under the emergency regulatory approval system [press release, 22 Nov 2022. https://www.shionogi.com/global/en/news/2022/11/e20221122.html. Accessed 17 Dec 2022.
-
- Shimizu R, Sonoyama T, Fukuhara T, Kuwata A, Matsuo Y, Kubota R. Safety, tolerability, and pharmacokinetics of the novel antiviral agent ensitrelvir fumaric acid, a SARS-CoV-2 3CL protease inhibitor, in healthy adults. Antimicrob Agents Chemother. 2022;66(10):e00632-e722. 10.1128/aac.00632-22. 10.1128/aac.00632-22 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

