Efficacy and Safety of Transitioning to Lemborexant from Z-drug, Suvorexant, and Ramelteon in Japanese Insomnia Patients: An Open-label, Multicenter Study
- PMID: 38460107
- PMCID: PMC10960898
- DOI: 10.1007/s12325-024-02811-2
Efficacy and Safety of Transitioning to Lemborexant from Z-drug, Suvorexant, and Ramelteon in Japanese Insomnia Patients: An Open-label, Multicenter Study
Abstract
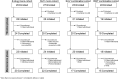
Introduction: For patients with chronic insomnia, conventional therapy may not always provide satisfactory efficacy and safety. Thus, switching to an alternative therapeutic agent can be explored. However, there is a lack of prospective studies evaluating the effectiveness of such changes. This prospective, non-randomized, open-label, interventional, multicenter study assessed whether Japanese patients with chronic insomnia dissatisfied with treatment could transition directly to lemborexant (LEM) from four cohorts-non-benzodiazepine sedative-hypnotic (zolpidem, zopiclone, or eszopiclone) monotherapy, dual orexin receptor antagonist (suvorexant) monotherapy, suvorexant + benzodiazepine receptor agonists (BZRAs), and melatonin receptor agonist (ramelteon) combination. We evaluated whether transitioning to LEM improved patient satisfaction based on efficacy and safety.
Methods: The primary endpoint was the proportion of successful transitions to LEM at 2 weeks (titration phase end), defined as the proportion of patients on LEM by the end of the 2-week titration phase who were willing to continue on LEM during the maintenance phase (Weeks 2-14). Patient satisfaction and safety (the incidence of treatment-emergent adverse events [TEAEs]) were assessed at 14 weeks (end of titration and maintenance phases).
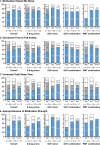
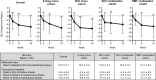
Results: Among the 90 patients enrolled, 95.6% (95% confidence interval: 89.0-98.8%) successfully transitioned to LEM at 2 weeks. The proportions of patients who successfully continued on LEM were 97.8% and 82.2% at the end of the titration and maintenance phases (Weeks 2 and 14), respectively. The overall incidence of TEAEs was 47.8%; no serious TEAEs occurred. In all cohorts, the proportions of patients with positive responses were higher than the proportions with negative responses on the three scales of the Patient Global Impression-Insomnia version. During the maintenance phase, Insomnia Severity Index scores generally improved at Weeks 2, 6, and 14 of LEM transition.
Conclusions: Direct transition to LEM may be a valid treatment option for patients with insomnia who are dissatisfied with current treatment.
Trial registration: ClinicalTrials.gov identifier, NCT04742699.
Keywords: Insomnia; Lemborexant; Ramelteon; Suvorexant; Switching; Z-drugs.
© 2024. The Author(s).
Conflict of interest statement
Motohiro Ozone received grants from Mitsubishi Tanabe Pharma Corporation, Tsumura Co., Ltd., Shionogi & Co., Ltd., Mochida Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., and Teijin Pharma Ltd.; and honoraria from Eisai Co., Ltd., Tsumura Co., Ltd., MSD Co., Ltd., Meiji Seika Pharma Co., Ltd., Takeda Pharmaceutical Company Limited, Nobelpharma Co., Ltd., Yoshitomi Pharmaceutical Industries, Ltd., and Mitsubishi Tanabe Pharma Corporation. Susumu Hirota received consulting fees from Viatris Inc.; honoraria from Eisai Co., Ltd., Meiji Seika Pharma Co., Ltd., Sumitomo Pharma Co., Ltd., and Takeda Pharmaceutical Company Ltd.; and is involved in clinical trials with Nippon Boehringer Ingelheim Co., Ltd., Shiongi & Co., Ltd., Sumitomo Pharma Co., Ltd., and Viatris Inc. Yu Ariyoshi received honoraria from Eisai Co., Ltd. and MSD Co., Ltd. Kenichi Hayashida received lecture fees from Eisai Co., Ltd. and MSD Co., Ltd. Azusa Ikegami received honoraria from Eisai Co., Ltd. Mitsunari Habukawa received honoraria from Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., MSD Co., Ltd., and Takeda Pharma Co., Ltd., Nozomu Kotorii received consulting fees from Eisai Co., Ltd., Takeda Pharmaceutical Company Limited, MSD Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., and Janssen Pharmaceutical K.K. Takehiro Taninaga is an employee of Eisai Co., Ltd. Naohisa Uchimura received honoraria from Eisai Co., Ltd., Meiji Seika Pharma Co., Ltd., MSD Co., Ltd., Nobelpharma Co., Ltd., and Takeda Pharmaceutical Company Limited. The remaining authors have no conflicts of interest to declare.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

