Blocking Ubiquitin-Specific Protease 7 Induces Ferroptosis in Gastric Cancer via Targeting Stearoyl-CoA Desaturase
- PMID: 38460164
- PMCID: PMC11095140
- DOI: 10.1002/advs.202307899
Blocking Ubiquitin-Specific Protease 7 Induces Ferroptosis in Gastric Cancer via Targeting Stearoyl-CoA Desaturase
Abstract
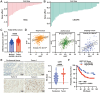
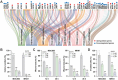
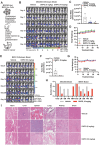
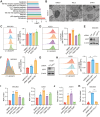
Gastric cancer (GC) presents a formidable global health challenge, and conventional therapies face efficacy limitations. Ubiquitin-specific protease 7 (USP7) plays pivotal roles in GC development, immune response, and chemo-resistance, making it a promising target. Various USP7 inhibitors have shown selectivity and efficacy in preclinical studies. However, the mechanistic role of USP7 has not been fully elucidated, and currently, no USP7 inhibitors have been approved for clinical use. In this study, DHPO is identified as a potent USP7 inhibitor for GC treatment through in silico screening. DHPO demonstrates significant anti-tumor activity in vitro, inhibiting cell viability and clonogenic ability, and preventing tumor migration and invasion. In vivo studies using orthotopic gastric tumor mouse models validate DHPO's efficacy in suppressing tumor growth and metastasis without significant toxicity. Mechanistically, DHPO inhibition triggers ferroptosis, evidenced by mitochondrial alterations, lipid Reactive Oxygen Species (ROS), Malondialdehyde (MDA) accumulation, and iron overload. Further investigations unveil USP7's regulation of Stearoyl-CoA Desaturase (SCD) through deubiquitination, linking USP7 inhibition to SCD degradation and ferroptosis induction. Overall, this study identifies USP7 as a key player in ferroptosis of GC, elucidates DHPO's inhibitory mechanisms, and highlights its potential for GC treatment by inducing ferroptosis through SCD regulation.
Keywords: SCD; USP7; USP7 inhibitor; ferroptosis; gastric cancer; ubiquitination.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Smyth E. C., Nilsson M., Grabsch H. I., van Grieken N. C., Lordick F., Lancet 2020, 396, 635. - PubMed
-
- Alsina M., Arrazubi V., Diez M., Tabernero J., Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 155. - PubMed
-
- Dewson G., Eichhorn P. J. A., Komander D., Nat. Rev. Cancer 2023, 23, 842. - PubMed
-
- Liu B., Ruan J., Chen M., Li Z., Manjengwa G., Schlüter D., Song W., Wang X., Mol. Psychiatry 2022, 27, 259. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
