Concentrated Solar Light Photoelectrochemical Water Splitting for Stable and High-Yield Hydrogen Production
- PMID: 38460173
- PMCID: PMC11234434
- DOI: 10.1002/advs.202309548
Concentrated Solar Light Photoelectrochemical Water Splitting for Stable and High-Yield Hydrogen Production
Abstract
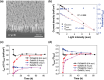
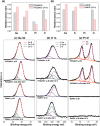
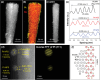
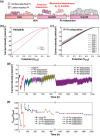
Photoelectrochemical water splitting is a promising technique for converting solar energy into low-cost and eco-friendly H2 fuel. However, the production rate of H2 is limited by the insufficient number of photogenerated charge carriers in the conventional photoelectrodes under 1 sun (100 mW cm-2) light. Concentrated solar light irradiation can overcome the issue of low yield, but it leads to a new challenge of stability because the accelerated reaction alters the surface chemical composition of photoelectrodes. Here, it is demonstrated that loading Pt nanoparticles (NPs) on single crystalline GaN nanowires (NWs) grown on n+-p Si photoelectrode operates efficiently and stably under concentrated solar light. Although a large number of Pt NPs detach during the initial reaction due to H2 gas bubbling, some Pt NPs which have an epitaxial relation with GaN NWs remain stably anchored. In addition, the stability of the photoelectrode further improves by redepositing Pt NPs on the reacted Pt/GaN surface, which results in maintaining onset potential >0.5 V versus reversible hydrogen electrode and photocurrent density >60 mA cm-2 for over 1500 h. The heterointerface between Pt cocatalysts and single crystalline GaN nanostructures shows great potential in designing an efficient and stable photoelectrode for high-yield solar to H2 conversion.
Keywords: concentrated solar light; hydrogen evolution; photoelectrochemical water splitting; stability.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
Some IP related to this work was licensed to NS Nanotech, Inc. and NX Fuels, Inc., which were co‐founded by Z. Mi. The University of Michigan and Mi have a financial interest in the companies.
Figures
References
-
- a) Paracchino A., Laporte V., Sivula K., Grätzel M., Thimsen E., Nat. Mater. 2011, 10, 456; - PubMed
- b) Bellani S., Antognazza M. R., Bonaccorso F., Adv. Mater. 2019, 31, 1801446; - PubMed
- c) Kaneko H., Minegishi T., Nakabayashi M., Shibata N., Kuang Y., Yamada T., Domen K., Adv. Funct. Mater. 2016, 26, 4570;
- d) Vanka S., Zhou B., Awni R. A., Song Z., Chowdhury F. A., Liu X., Hajibabaei H., Shi W., Xiao Y., Navid I. A., ACS Energy Lett. 2020, 5, 3741.
-
- Jin M., Zhang X., Niu S., Wang Q., Huang R., Ling R., Huang J., Shi R., Amini A., Cheng C., ACS Nano 2022, 16, 11577. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
