Purinergic signaling promotes premature senescence
- PMID: 38460941
- PMCID: PMC11002311
- DOI: 10.1016/j.jbc.2024.107145
Purinergic signaling promotes premature senescence
Abstract
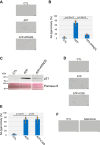
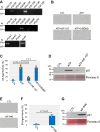
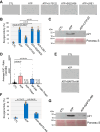
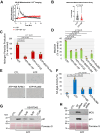
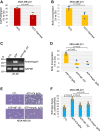
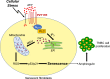
Extracellular ATP activates P2 purinergic receptors. Whether purinergic signaling is functionally coupled to cellular senescence is largely unknown. We find that oxidative stress induced release of ATP and caused senescence in human lung fibroblasts. Inhibition of P2 receptors limited oxidative stress-induced senescence, while stimulation with exogenous ATP promoted premature senescence. Pharmacological inhibition of P2Y11 receptor (P2Y11R) inhibited premature senescence induced by either oxidative stress or ATP, while stimulation with a P2Y11R agonist was sufficient to induce cellular senescence. Our data show that both extracellular ATP and a P2Y11R agonist induced calcium (Ca++) release from the endoplasmic reticulum (ER) and that either inhibition of phospholipase C or intracellular Ca++ chelation impaired ATP-induced senescence. We also find that Ca++ that was released from the ER, following ATP-mediated activation of phospholipase C, entered mitochondria in a manner dependent on P2Y11R activation. Once in mitochondria, excessive Ca++ promoted the production of reactive oxygen species in a P2Y11R-dependent fashion, which drove development of premature senescence of lung fibroblasts. Finally, we show that conditioned medium derived from senescent lung fibroblasts, which were induced to senesce through the activation of ATP/P2Y11R-mediated signaling, promoted the proliferation of triple-negative breast cancer cells and their tumorigenic potential by secreting amphiregulin. Our study identifies the existence of a novel purinergic signaling pathway that links extracellular ATP to the development of a protumorigenic premature senescent phenotype in lung fibroblasts that is dependent on P2Y11R activation and ER-to-mitochondria calcium signaling.
Keywords: ATP; purinergic signaling; reactive oxygen species; senescence; triple negative breast cancer cells.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Lundberg A.S., Hahn W.C., Gupta P., Weinberg R.A. Genes involved in senescence and immortalization. Curr. Opin. Cell Biol. 2000;12:705–709. - PubMed
-
- Sherr C.J., DePinho R.A. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407–410. - PubMed
-
- Wynford-Thomas D. Cellular senescence and cancer. J. Pathol. 1999;187:100–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

