MicroRNA-18a prevents senescence of mesenchymal stem cells by targeting CTDSPL
- PMID: 38460957
- PMCID: PMC10968691
- DOI: 10.18632/aging.205642
MicroRNA-18a prevents senescence of mesenchymal stem cells by targeting CTDSPL
Abstract
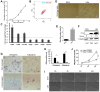
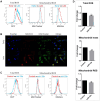
Stem cell therapy requires massive-scale homogeneous stem cells under strict qualification control. However, Prolonged ex vivo expansion impairs the biological functions and results in senescence of mesenchymal stem cells (MSCs). We investigated the function of CTDSPL in the premature senescence process of MSCs and clarified that miR-18a-5p played a prominent role in preventing senescence of long-term cultured MSCs and promoting the self-renewal ability of MSCs. Over-expression of CTDSPL resulted in an enlarged morphology, up-regulation of p16 and accumulation of SA-β-gal of MSCs. The reduced phosphorylated RB suggested cell cycle arrest of MSCs. All these results implied that CTDSPL induced premature senescence of MSCs. We further demonstrated that miR-18a-5p was a putative regulator of CTDSPL by luciferase reporter assay. Inhibition of miR-18a-5p promoted the expression of CTDSPL and induced premature senescence of MSCs. Continuous overexpression of miR-18a-5p improved self-renewal of MSCs by reducing ROS level, increased expression of Oct4 and Nanog, and promoted growth rate and differentiation capability. We reported for the first time that the dynamic interaction of miR-18a-5p and CTDSPL is crucial for stem cell senescence.
Keywords: CTDSPL; mesenchymal stem cells; miR-18a-5p; senescence.
Conflict of interest statement
Figures




Similar articles
-
miR-155-5p inhibition rejuvenates aged mesenchymal stem cells and enhances cardioprotection following infarction.Aging Cell. 2020 Apr;19(4):e13128. doi: 10.1111/acel.13128. Epub 2020 Mar 20. Aging Cell. 2020. PMID: 32196916 Free PMC article.
-
miR-29c-3p promotes senescence of human mesenchymal stem cells by targeting CNOT6 through p53-p21 and p16-pRB pathways.Biochim Biophys Acta. 2016 Apr;1863(4):520-32. doi: 10.1016/j.bbamcr.2016.01.005. Epub 2016 Jan 11. Biochim Biophys Acta. 2016. PMID: 26792405
-
Inhibition of miR-199a-5p rejuvenates aged mesenchymal stem cells derived from patients with idiopathic pulmonary fibrosis and improves their therapeutic efficacy in experimental pulmonary fibrosis.Stem Cell Res Ther. 2021 Feb 25;12(1):147. doi: 10.1186/s13287-021-02215-x. Stem Cell Res Ther. 2021. PMID: 33632305 Free PMC article.
-
The p53/miR-145a Axis Promotes Cellular Senescence and Inhibits Osteogenic Differentiation by Targeting Cbfb in Mesenchymal Stem Cells.Front Endocrinol (Lausanne). 2021 Jan 11;11:609186. doi: 10.3389/fendo.2020.609186. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33505358 Free PMC article.
-
miR-18a-5p derived from mesenchymal stem cells-extracellular vesicles inhibits ovarian cancer cell proliferation, migration, invasion, and chemotherapy resistance.J Transl Med. 2022 Jun 7;20(1):258. doi: 10.1186/s12967-022-03422-7. J Transl Med. 2022. PMID: 35672774 Free PMC article.
References
-
- Abushouk AI, Salem AMA, Saad A, Afifi AM, Afify AY, Afify H, Salem HSE, Ghanem E, Abdel-Daim MM. Mesenchymal Stem Cell Therapy for Doxorubicin-Induced Cardiomyopathy: Potential Mechanisms, Governing Factors, and Implications of the Heart Stem Cell Debate. Front Pharmacol. 2019; 10:635. 10.3389/fphar.2019.00635 - DOI - PMC - PubMed
-
- Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lässer C, Segaliny AI, McIntyre LL, Shelke GV, Hutchins E, Hamamoto A, Calle EN, Crescitelli R, Liao W, et al.. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano. 2019; 13:6670–88. 10.1021/acsnano.9b01004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

