Molecular characterization of Golgi apparatus-related genes indicates prognosis and immune infiltration in osteosarcoma
- PMID: 38460960
- PMCID: PMC11006476
- DOI: 10.18632/aging.205645
Molecular characterization of Golgi apparatus-related genes indicates prognosis and immune infiltration in osteosarcoma
Abstract
Background: The Golgi apparatus (GA) is crucial for protein synthesis and modification, and regulates various cellular processes. Dysregulation of GA can lead to pathological conditions like neoplastic growth. GA-related genes (GARGs) mutations are commonly found in cancer, contributing to tumor metastasis. However, the expression and prognostic significance of GARGs in osteosarcoma are yet to be understood.
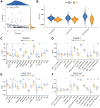
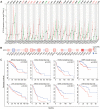
Methods: Gene expression and clinical data of osteosarcoma patients were obtained from the TARGET and GEO databases. A consensus clustering analysis identified distinct molecular subtypes based on GARGs. Discrepancies in biological processes and immunological features among the subtypes were explored using GSVA, ssGSEA, and Metascape analysis. A GARGs signature was constructed using Cox regression. The prognostic value of the GARGs signature in osteosarcoma was evaluated using Kaplan-Meier curves and a nomogram.
Results: Two GARG subtypes were identified, with Cluster A showing better prognosis, immunogenicity, and immune cell infiltration than Cluster B. A novel risk model of 3 GARGs was established using the TARGET dataset and validated with independent datasets. High-risk patients had poorer overall survival, and the GARGs signature independently predicted osteosarcoma prognosis. Combining risk scores and clinical characteristics in a nomogram improved prediction performance. Additionally, we discovered Stanniocalcin-2 (STC2) as a significant prognostic gene highly expressed in osteosarcoma and potential disease biomarker.
Conclusions: Our study revealed that patients with osteosarcoma can be divided into two GARGs subgroups. Furthermore, we have developed a GARGs prognostic signature that can accurately forecast the prognosis of osteosarcoma patients.
Keywords: Golgi apparatus; biomarkers; immune infiltration; osteosarcoma; prognosis.
Conflict of interest statement
Figures






References
-
- Kelley LM, Schlegel M, Hecker-Nolting S, Kevric M, Haller B, Rössig C, Reichardt P, Kager L, Kühne T, Gosheger G, Windhager R, Specht K, Rechl H, et al. Pathological Fracture and Prognosis of High-Grade Osteosarcoma of the Extremities: An Analysis of 2,847 Consecutive Cooperative Osteosarcoma Study Group (COSS) Patients. J Clin Oncol. 2020; 38:823–33. 10.1200/JCO.19.00827 - DOI - PubMed
-
- Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC, Anninga J, Antal I, Arndt C, Brown KLB, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019; 109:36–50. 10.1016/j.ejca.2018.11.027 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

