Inhaled corticosteroids and Stenotrophomonas maltophilia in outpatients with chronic obstructive pulmonary disease: a retrospective cohort study
- PMID: 38460975
- PMCID: PMC10928788
- DOI: 10.1136/bmjresp-2023-001929
Inhaled corticosteroids and Stenotrophomonas maltophilia in outpatients with chronic obstructive pulmonary disease: a retrospective cohort study
Abstract
Objectives: Inhaled corticosteroids (ICS) are widely used in patients with chronic obstructive pulmonary disease (COPD). However, ICS are associated with an increased risk of adverse effects.We aimed to determine whether an association between a lower respiratory tract culture with Stenotrophomonas maltophilia and increasing ICS dosing in patients with COPD exists.
Design: An observational cohort study of outpatients with COPD in Denmark between 2010 and 2018.ICS exposure was categorised into four groups based on average daily consumption 1 year prior to inclusion: no use, low ICS dose (≤400 µg), moderate ICS dose (400-800 µg) and high ICS dose (>800 µg). Dose-response relationship was investigated by a multivariable Cox proportional hazards regression.
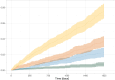
Results: Of the total 22 689 patients, 459 had lower respiratory tract cultures positive for S. maltophilia. The HR of S. maltophilia increased with increasing daily ICS dose: low ICS dose HR 2.6 (95% CI 1.6 to 4.0), moderate ICS dose HR 3.0 (95% CI 1.9 to 4.6) and high ICS dose HR 5.7 (95% CI 3.8 to 8.5).
Conclusions: We found that ICS was associated with a high, dose-dependent increased hazard of S. maltophilia in outpatients with COPD. High dose users had a nearly six times increased hazard compared with non-users of ICS. When appropriate, attempts at de-escalating ICS treatment should be made.
Keywords: COPD Pathology; COPD epidemiology; Pulmonary Disease, Chronic Obstructive.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Outside the submitted work, RBD has been on an advisory board for Pfizer. CSU has received grants from Sanofi, Boehringer Ingelheim, AstraZeneca and Novartis; consulting fees from Chiesi, Orion Pharma, AstraZeneca, GSK and TEVA; speaker fees from Orion Pharma, AstraZeneca and TEVA; and been on advisory boards for Novartis, Sanofi, Glaxo-Smith Kline, Chiesi, AstraZeneca and Boehringer Ingelheim.
Figures
Similar articles
-
Hospitalization for chronic obstructive pulmonary disease and pneumonia: association with the dose of inhaled corticosteroids. A nation-wide cohort study of 52 100 outpatients.Clin Microbiol Infect. 2023 Apr;29(4):523-529. doi: 10.1016/j.cmi.2022.11.029. Epub 2022 Dec 8. Clin Microbiol Infect. 2023. PMID: 36503112
-
Mortality and exacerbations associated with Stenotrophomonas maltophilia in chronic obstructive pulmonary disease. A regional cohort study of 22,689 outpatients.Respir Res. 2023 Sep 26;24(1):232. doi: 10.1186/s12931-023-02544-w. Respir Res. 2023. PMID: 37752596 Free PMC article.
-
Effects of inhaled corticosteroids /long-acting agonists in a single inhaler versus inhaled corticosteroids alone on all-cause mortality, pneumonia, and fracture in chronic obstructive pulmonary disease: A nationwide cohort study 2002-2013.Respir Med. 2017 Sep;130:75-84. doi: 10.1016/j.rmed.2017.07.012. Epub 2017 Jul 19. Respir Med. 2017. PMID: 29206637
-
Absolute Blood Eosinophil Counts to Guide Inhaled Corticosteroids Therapy Among Patients with COPD: Systematic Review and Meta-analysis.Curr Drug Targets. 2019;20(16):1670-1679. doi: 10.2174/1389450120666190808141625. Curr Drug Targets. 2019. PMID: 31393244
-
Overuse of long-acting β2-agonist/inhaled corticosteroids in patients with chronic obstructive pulmonary disease: time to rethink prescribing patterns.Postgrad Med. 2023 Nov;135(8):784-802. doi: 10.1080/00325481.2023.2284650. Epub 2024 Jan 10. Postgrad Med. 2023. PMID: 38032494 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
