In vivo phenotypic vascular dysfunction extends beyond the aorta in a mouse model for fibrillin-1 (Fbn1) mutation
- PMID: 38461168
- PMCID: PMC10924961
- DOI: 10.1038/s41598-024-56438-y
In vivo phenotypic vascular dysfunction extends beyond the aorta in a mouse model for fibrillin-1 (Fbn1) mutation
Abstract
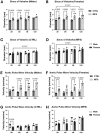
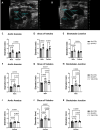
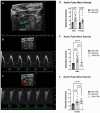
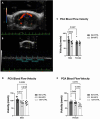
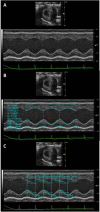
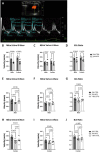
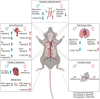
In individuals with Marfan Syndrome (MFS), fibrillin-1 gene (FBN1) mutations can lead to vascular wall weakening and dysfunction. The experimental mouse model of MFS (Fbn1C1041G/+) has been advantageous in investigating MFS-associated life-threatening aortic aneurysms. It is well established that the MFS mouse model exhibits an accelerated-aging phenotype in elastic organs like the aorta, lung, and skin. However, the impact of Fbn1 mutations on the in vivo function and structure of various artery types with the consideration of sex and age, has not been adequately explored in real-time and a clinically relevant context. In this study, we investigate if Fbn1 mutation contributes to sex-dependent alterations in central and cerebral vascular function similar to phenotypic changes associated with normal aging in healthy control mice. In vivo ultrasound imaging of central and cerebral vasculature was performed in 6-month-old male and female MFS and C57BL/6 mice and sex-matched 12-month-old (middle-aged) healthy control mice. Our findings confirm aortic enlargement (aneurysm) and wall stiffness in MFS mice, but with exacerbation in male diameters. Coronary artery blood flow velocity (BFV) in diastole was not different but left pulmonary artery BFV was decreased in MFS and 12-month-old control mice regardless of sex. At 6 months of age, MFS male mice show decreased posterior cerebral artery BFV as compared to age-matched control males, with no difference observed between female cohorts. Reduced mitral valve early-filling velocities were indicated in MFS mice regardless of sex. Male MFS mice also demonstrated left ventricular hypertrophy. Overall, these results underscore the significance of biological sex in vascular function and structure in MFS mice, while highlighting a trend of pre-mature vascular aging phenotype in MFS mice that is comparable to phenotypes observed in older healthy controls. Furthermore, this research is a vital step in understanding MFS's broader implications and sets the stage for more in-depth future analyses, while providing data-driven preclinical justification for re-evaluating diagnostic approaches and therapeutic efficacy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
In Vivo Phenotypic Vascular Dysfunction Extends Beyond the Aorta in a Mouse Model for Fibrillin-1 ( FBN1 ) Mutation.bioRxiv [Preprint]. 2023 Nov 18:2023.11.18.567641. doi: 10.1101/2023.11.18.567641. bioRxiv. 2023. Update in: Sci Rep. 2024 Mar 9;14(1):5779. doi: 10.1038/s41598-024-56438-y. PMID: 38014144 Free PMC article. Updated. Preprint.
Similar articles
-
In Vivo Phenotypic Vascular Dysfunction Extends Beyond the Aorta in a Mouse Model for Fibrillin-1 ( FBN1 ) Mutation.bioRxiv [Preprint]. 2023 Nov 18:2023.11.18.567641. doi: 10.1101/2023.11.18.567641. bioRxiv. 2023. Update in: Sci Rep. 2024 Mar 9;14(1):5779. doi: 10.1038/s41598-024-56438-y. PMID: 38014144 Free PMC article. Updated. Preprint.
-
Single-Cell Transcriptomic Profiling of Vascular Smooth Muscle Cell Phenotype Modulation in Marfan Syndrome Aortic Aneurysm.Arterioscler Thromb Vasc Biol. 2020 Sep;40(9):2195-2211. doi: 10.1161/ATVBAHA.120.314670. Epub 2020 Jul 23. Arterioscler Thromb Vasc Biol. 2020. PMID: 32698686 Free PMC article.
-
Cardiovascular characteristics in Marfan syndrome and their relation to the genotype.Verh K Acad Geneeskd Belg. 2009;71(6):335-71. Verh K Acad Geneeskd Belg. 2009. PMID: 20232788 Review.
-
Age- and sex-specific biomechanics and extracellular matrix remodeling of the ascending aorta in a mouse model of severe Marfan syndrome.Am J Physiol Heart Circ Physiol. 2024 Oct 1;327(4):H1037-H1051. doi: 10.1152/ajpheart.00391.2024. Epub 2024 Aug 30. Am J Physiol Heart Circ Physiol. 2024. PMID: 39212766
-
A novel fibrillin-1 gene missense mutation associated with neonatal Marfan syndrome: a case report and review of the mutation spectrum.BMC Pediatr. 2016 Apr 30;16:60. doi: 10.1186/s12887-016-0598-6. BMC Pediatr. 2016. PMID: 27138491 Free PMC article. Review.
Cited by
-
Modification of extracellular matrix proteins by oxidants and electrophiles.Biochem Soc Trans. 2024 Jun 26;52(3):1199-1217. doi: 10.1042/BST20230860. Biochem Soc Trans. 2024. PMID: 38778764 Free PMC article. Review.
-
Exploring thoracic aorta ECM alterations in Marfan syndrome: insights into aorta wall structure.Sci Rep. 2025 Jul 22;15(1):26665. doi: 10.1038/s41598-025-09665-w. Sci Rep. 2025. PMID: 40695874 Free PMC article.
-
Cerebral microvascular density, blood-brain barrier permeability, and support for neuroinflammation indicate early aging in a Marfan syndrome mouse model.Front Physiol. 2025 Jan 31;15:1457034. doi: 10.3389/fphys.2024.1457034. eCollection 2024. Front Physiol. 2025. PMID: 39959812 Free PMC article.
-
Sex- and age-dependent neurovascular abnormalities linked to neuroinflammation lead to exacerbated post-ischemic brain injury in Marfan syndrome mice.Redox Biol. 2025 Jun;83:103662. doi: 10.1016/j.redox.2025.103662. Epub 2025 May 7. Redox Biol. 2025. PMID: 40349485 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

